Prolongevity hormone FGF21 protects against immune senescence by delaying age-related thymic involution
- PMID: 26755598
- PMCID: PMC4743827
- DOI: 10.1073/pnas.1514511113
Prolongevity hormone FGF21 protects against immune senescence by delaying age-related thymic involution
Abstract
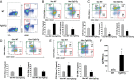
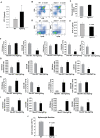
Age-related thymic degeneration is associated with loss of naïve T cells, restriction of peripheral T-cell diversity, and reduced healthspan due to lower immune competence. The mechanistic basis of age-related thymic demise is unclear, but prior evidence suggests that caloric restriction (CR) can slow thymic aging by maintaining thymic epithelial cell integrity and reducing the generation of intrathymic lipid. Here we show that the prolongevity ketogenic hormone fibroblast growth factor 21 (FGF21), a member of the endocrine FGF subfamily, is expressed in thymic stromal cells along with FGF receptors and its obligate coreceptor, βKlotho. We found that FGF21 expression in thymus declines with age and is induced by CR. Genetic gain of FGF21 function in mice protects against age-related thymic involution with an increase in earliest thymocyte progenitors and cortical thymic epithelial cells. Importantly, FGF21 overexpression reduced intrathymic lipid, increased perithymic brown adipose tissue, and elevated thymic T-cell export and naïve T-cell frequencies in old mice. Conversely, loss of FGF21 function in middle-aged mice accelerated thymic aging, increased lethality, and delayed T-cell reconstitution postirradiation and hematopoietic stem cell transplantation (HSCT). Collectively, FGF21 integrates metabolic and immune systems to prevent thymic injury and may aid in the reestablishment of a diverse T-cell repertoire in cancer patients following HSCT.
Keywords: FGF21; aging; inflammation; metabolism; thymus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Immunometabolism: Unravelling the puzzle to longevity and immunity.Nat Rev Immunol. 2016 Feb;16(2):74-5. doi: 10.1038/nri.2016.16. Nat Rev Immunol. 2016. PMID: 26831525 No abstract available.
Similar articles
-
Enhanced paracrine action of FGF21 in stromal cells delays thymic aging.Nat Aging. 2025 Apr;5(4):576-587. doi: 10.1038/s43587-025-00813-5. Epub 2025 Feb 19. Nat Aging. 2025. PMID: 39972172 Free PMC article.
-
Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution.J Immunol. 2009 Sep 1;183(5):3040-52. doi: 10.4049/jimmunol.0900562. Epub 2009 Jul 31. J Immunol. 2009. PMID: 19648267 Free PMC article.
-
Thymic microenvironment's impact on immunosenescence.Immunol Res. 2024 Oct;72(5):1161-1173. doi: 10.1007/s12026-024-09519-z. Epub 2024 Jul 23. Immunol Res. 2024. PMID: 39042204
-
Thymic Engraftment by in vitro-Derived Progenitor T Cells in Young and Aged Mice.Front Immunol. 2020 Aug 18;11:1850. doi: 10.3389/fimmu.2020.01850. eCollection 2020. Front Immunol. 2020. PMID: 32973763 Free PMC article. Review.
-
Impact of immune-metabolic interactions on age-related thymic demise and T cell senescence.Semin Immunol. 2012 Oct;24(5):321-30. doi: 10.1016/j.smim.2012.04.002. Epub 2012 Apr 28. Semin Immunol. 2012. PMID: 22546243 Review.
Cited by
-
Metabolic Messengers: FGF21.Nat Metab. 2021 Mar;3(3):309-317. doi: 10.1038/s42255-021-00354-2. Epub 2021 Mar 18. Nat Metab. 2021. PMID: 33758421 Free PMC article. Review.
-
Endothelial senescence in vascular diseases: current understanding and future opportunities in senotherapeutics.Exp Mol Med. 2023 Jan;55(1):1-12. doi: 10.1038/s12276-022-00906-w. Epub 2023 Jan 4. Exp Mol Med. 2023. PMID: 36599934 Free PMC article. Review.
-
Brain-specific PAPP-A knock-out mice?Exp Gerontol. 2021 Oct 15;154:111548. doi: 10.1016/j.exger.2021.111548. Epub 2021 Sep 9. Exp Gerontol. 2021. PMID: 34509589 Free PMC article.
-
Sodium nitrate regulates senescence accompanied by aortic atherosclerosis in ApoE-/- mice through the miR-34a/FGF-21 axis.Front Pharmacol. 2025 Mar 5;16:1562321. doi: 10.3389/fphar.2025.1562321. eCollection 2025. Front Pharmacol. 2025. PMID: 40110129 Free PMC article.
-
Exploring Lymph Node Stroma Ageing: Immune Implications and Future Directions.Aging Cell. 2025 Mar;24(3):e70000. doi: 10.1111/acel.70000. Epub 2025 Feb 15. Aging Cell. 2025. PMID: 39954244 Free PMC article. Review.
References
-
- Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: Is it ever too old to become young again? Nat Rev Immunol. 2009;9(1):57–62. - PubMed
-
- Dixit VD. Impact of immune-metabolic interactions on age-related thymic demise and T cell senescence. Semin Immunol. 2012;24(5):321–330. - PubMed
-
- Heng TS, Chidgey AP, Boyd RL. Getting back at nature: Understanding thymic development and overcoming its atrophy. Curr Opin Pharmacol. 2010;10(4):425–433. - PubMed
-
- van Ewijk W. T-cell differentiation is influenced by thymic microenvironments. Annu Rev Immunol. 1991;9:591–615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

