Attention promotes episodic encoding by stabilizing hippocampal representations
- PMID: 26755611
- PMCID: PMC4743819
- DOI: 10.1073/pnas.1518931113
Attention promotes episodic encoding by stabilizing hippocampal representations
Abstract
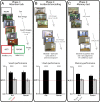
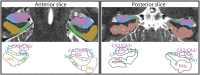
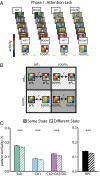
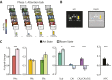
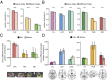
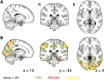
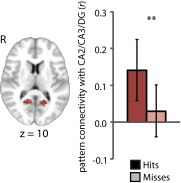
Attention influences what is later remembered, but little is known about how this occurs in the brain. We hypothesized that behavioral goals modulate the attentional state of the hippocampus to prioritize goal-relevant aspects of experience for encoding. Participants viewed rooms with paintings, attending to room layouts or painting styles on different trials during high-resolution functional MRI. We identified template activity patterns in each hippocampal subfield that corresponded to the attentional state induced by each task. Participants then incidentally encoded new rooms with art while attending to the layout or painting style, and memory was subsequently tested. We found that when task-relevant information was better remembered, the hippocampus was more likely to have been in the correct attentional state during encoding. This effect was specific to the hippocampus, and not found in medial temporal lobe cortex, category-selective areas of the visual system, or elsewhere in the brain. These findings provide mechanistic insight into how attention transforms percepts into memories.
Keywords: hippocampal subfields; long-term memory; medial temporal lobe; representational stability; selective attention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

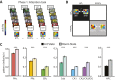
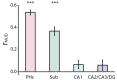
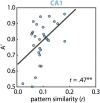
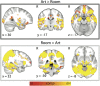



References
-
- Chun MM, Golomb JD, Turk-Browne NB. A taxonomy of external and internal attention. Annu Rev Psychol. 2011;62:73–101. - PubMed
-
- Maunsell JHR, Treue S. Feature-based attention in visual cortex. Trends Neurosci. 2006;29(6):317–322. - PubMed
-
- Chun MM, Turk-Browne NB. Interactions between attention and memory. Curr Opin Neurobiol. 2007;17(2):177–184. - PubMed
-
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: Insights from relational and item-based learning. J Neurophysiol. 2002;88(2):982–990. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

