What is going on between defibrotide and endothelial cells? Snapshots reveal the hot spots of their romance
- PMID: 26755708
- PMCID: PMC4817313
- DOI: 10.1182/blood-2015-10-676114
What is going on between defibrotide and endothelial cells? Snapshots reveal the hot spots of their romance
Abstract
Defibrotide (DF) has received European Medicines Agency authorization to treat sinusoidal obstruction syndrome, an early complication after hematopoietic cell transplantation. DF has a recognized role as an endothelial protective agent, although its precise mechanism of action remains to be elucidated. The aim of the present study was to investigate the interaction of DF with endothelial cells (ECs). A human hepatic EC line was exposed to different DF concentrations, previously labeled. Using inhibitory assays and flow cytometry techniques along with confocal microscopy, we explored: DF-EC interaction, endocytic pathways, and internalization kinetics. Moreover, we evaluated the potential role of adenosine receptors in DF-EC interaction and if DF effects on endothelium were dependent of its internalization. Confocal microscopy showed interaction of DF with EC membranes followed by internalization, though DF did not reach the cell nucleus even after 24 hours. Flow cytometry revealed concentration, temperature, and time dependent uptake of DF in 2 EC models but not in other cell types. Moreover, inhibitory assays indicated that entrance of DF into ECs occurs primarily through macropinocytosis. Our experimental approach did not show any evidence of the involvement of adenosine receptors in DF-EC interaction. The antiinflammatory and antioxidant properties of DF seem to be caused by the interaction of the drug with the cell membrane. Our findings contribute to a better understanding of the precise mechanisms of action of DF as a therapeutic and potential preventive agent on the endothelial damage underlying different pathologic situations.
© 2016 by The American Society of Hematology.
Figures




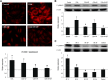
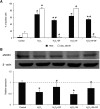
Comment in
-
Long-awaited news for hepatic veno-occlusive disease.Blood. 2016 Mar 31;127(13):1630-1. doi: 10.1182/blood-2016-02-694943. Blood. 2016. PMID: 27034420
References
-
- Richardson PG, Corbacioglu S, Ho VT, et al. Drug safety evaluation of defibrotide. Expert Opin Drug Saf. 2013;12(1):123–136. - PubMed
-
- Espinosa G, Berman H, Cervera R. Management of refractory cases of catastrophic antiphospholipid syndrome. Autoimmun Rev. 2011;10(11):664–668. - PubMed
-
- Pescador R, Capuzzi L, Mantovani M, Fulgenzi A, Ferrero ME. Defibrotide: properties and clinical use of an old/new drug. Vascul Pharmacol. 2013;59(1-2):1–10. - PubMed
-
- Palomo M, Diaz-Ricart M, Carbo C, et al. The release of soluble factors contributing to endothelial activation and damage after hematopoietic stem cell transplantation is not limited to the allogeneic setting and involves several pathogenic mechanisms. Biol Blood Marrow Transplant. 2009;15(5):537–546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

