Long-acting recombinant coagulation factor IX albumin fusion protein (rIX-FP) in hemophilia B: results of a phase 3 trial
- PMID: 26755710
- PMCID: PMC4825413
- DOI: 10.1182/blood-2015-09-669234
Long-acting recombinant coagulation factor IX albumin fusion protein (rIX-FP) in hemophilia B: results of a phase 3 trial
Abstract
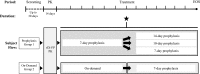
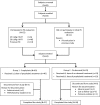
A global phase 3 study evaluated the pharmacokinetics, efficacy, and safety of recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in 63 previously treated male patients (12-61 years) with severe hemophilia B (factor IX [FIX] activity ≤2%). The study included 2 groups: group 1 patients received routine prophylaxis once every 7 days for 26 weeks, followed by either 7-, 10-, or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively; group 2 patients received on-demand treatment of bleeding episodes for 26 weeks and then switched to a 7-day prophylaxis regimen for a mean of 45 weeks. The mean terminal half-life of rIX-FP was 102 hours, 4.3-fold longer than previous FIX treatment. Patients maintained a mean trough of 20 and 12 IU/dL FIX activity on prophylaxis with rIX-FP 40 IU/kg weekly and 75 IU/kg every 2 weeks, respectively. There was 100% reduction in median annualized spontaneous bleeding rate (AsBR) and 100% resolution of target joints when subjects switched from on-demand to prophylaxis treatment with rIX-FP (P< .0001). The median AsBR was 0.00 for all prophylaxis regimens. Overall, 98.6% of bleeding episodes were treated successfully, including 93.6% that were treated with a single injection. No patient developed an inhibitor, and no safety concerns were identified. These results indicate rIX-FP is safe and effective for preventing and treating bleeding episodes in patients with hemophilia B at dosing regimens of 40 IU/kg weekly and 75 IU/kg every 2 weeks. This trial was registered at www.clinicaltrials.gov as #NCT0101496274.
Trial registration: ClinicalTrials.gov NCT01496274.
© 2016 by The American Society of Hematology.
Figures

Comment in
-
A new era for hemophilia B treatment.Blood. 2016 Apr 7;127(14):1734-6. doi: 10.1182/blood-2016-02-694869. Blood. 2016. PMID: 27056991 Free PMC article.
References
-
- Bolton-Maggs PHB, Pasi KJ. Haemophilias A and B. Lancet. 2003;361(9371):1801–1809. - PubMed
-
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Treatment Guidelines Working Group on Behalf of The World Federation Of Hemophilia. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–e47. - PubMed
-
- Baxter Healthcare Corporation. RIXUBIS (package insert). http://www.rixubis.com/pdf/rixubis_pi.pdf. Accessed September 1, 2015.
-
- Biogen Idec Inc. ALPROLIX (package insert). http://www.alprolix.com/pdfs/PrescribingInformation.pdf. Accessed September 1, 2015.
-
- Powell JS, Pasi KJ, Ragni MV, et al. B-LONG Investigators. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313–2323. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

