Catalysts for CO2/epoxide ring-opening copolymerization
- PMID: 26755758
- PMCID: PMC4707689
- DOI: 10.1098/rsta.2015.0085
Catalysts for CO2/epoxide ring-opening copolymerization
Abstract
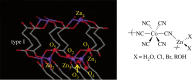
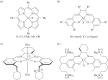
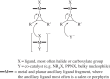
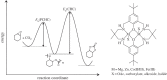
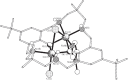
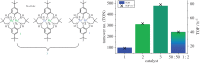
This article summarizes and reviews recent progress in the development of catalysts for the ring-opening copolymerization of carbon dioxide and epoxides. The copolymerization is an interesting method to add value to carbon dioxide, including from waste sources, and to reduce pollution associated with commodity polymer manufacture. The selection of the catalyst is of critical importance to control the composition, properties and applications of the resultant polymers. This review highlights and exemplifies some key recent findings and hypotheses, in particular using examples drawn from our own research.
Keywords: CO2; catalysis; polycarbonate; ring-opening copolymerization.
© 2016 The Authors.
Figures
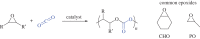




References
-
- Inoue S, Koinuma H, Tsuruta T. 1969. Copolymerization of carbon dioxide and epoxide. J. Poly. Sci. B Polym. Lett. 7, 287–292. (10.1002/pol.1969.110070408) - DOI
-
- Luinstra GA. 2008. Poly(propylene carbonate), old copolymers of propylene oxide and carbon dioxide with new interests: catalysis and material properties. Polym. Rev. 48, 192–219. (10.1080/15583720701834240) - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
