Clonal Analysis of Newborn Hippocampal Dentate Granule Cell Proliferation and Development in Temporal Lobe Epilepsy
- PMID: 26756038
- PMCID: PMC4706641
- DOI: 10.1523/ENEURO.0087-15.2015
Clonal Analysis of Newborn Hippocampal Dentate Granule Cell Proliferation and Development in Temporal Lobe Epilepsy
Abstract
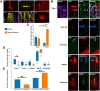
Hippocampal dentate granule cells are among the few neuronal cell types generated throughout adult life in mammals. In the normal brain, new granule cells are generated from progenitors in the subgranular zone and integrate in a typical fashion. During the development of epilepsy, granule cell integration is profoundly altered. The new cells migrate to ectopic locations and develop misoriented "basal" dendrites. Although it has been established that these abnormal cells are newly generated, it is not known whether they arise ubiquitously throughout the progenitor cell pool or are derived from a smaller number of "bad actor" progenitors. To explore this question, we conducted a clonal analysis study in mice expressing the Brainbow fluorescent protein reporter construct in dentate granule cell progenitors. Mice were examined 2 months after pilocarpine-induced status epilepticus, a treatment that leads to the development of epilepsy. Brain sections were rendered translucent so that entire hippocampi could be reconstructed and all fluorescently labeled cells identified. Our findings reveal that a small number of progenitors produce the majority of ectopic cells following status epilepticus, indicating that either the affected progenitors or their local microenvironments have become pathological. By contrast, granule cells with "basal" dendrites were equally distributed among clonal groups. This indicates that these progenitors can produce normal cells and suggests that global factors sporadically disrupt the dendritic development of some new cells. Together, these findings strongly predict that distinct mechanisms regulate different aspects of granule cell pathology in epilepsy.
Keywords: adult neurogenesis; clonal analysis; dentate granule cells; epilepsy; pilocarpine; progenitor cells.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
