Chain Walking of Allylrhodium Species Towards Esters During Rhodium-Catalyzed Nucleophilic Allylations of Imines
- PMID: 26756445
- PMCID: PMC4736453
- DOI: 10.1002/anie.201508964
Chain Walking of Allylrhodium Species Towards Esters During Rhodium-Catalyzed Nucleophilic Allylations of Imines
Abstract
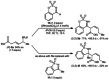
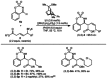
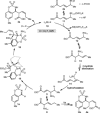
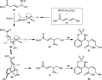
Allylrhodium species derived from δ-trifluoroboryl β,γ-unsaturated esters undergo chain walking towards the ester moiety. The resulting allylrhodium species react with imines to give products containing two new stereocenters and a Z-alkene. By using a chiral diene ligand, products can be obtained with high enantioselectivities, where a pronounced matched/mismatched effect with the chirality of the allyltrifluoroborate is evident.
Keywords: allyltrifluoroborates; asymmetric catalysis; imines; isomerization; rhodium.
© 2015 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures



Similar articles
-
Enantioselective Rhodium-Catalyzed Coupling of Arylboronic Acids, 1,3-Enynes, and Imines by Alkenyl-to-Allyl 1,4-Rhodium(I) Migration.Angew Chem Int Ed Engl. 2017 Dec 18;56(51):16352-16356. doi: 10.1002/anie.201709334. Epub 2017 Nov 24. Angew Chem Int Ed Engl. 2017. PMID: 28980437 Free PMC article.
-
Arylative Intramolecular Allylation of Ketones with 1,3-Enynes Enabled by Catalytic Alkenyl-to-Allyl 1,4-Rhodium(I) Migration.Angew Chem Int Ed Engl. 2017 Jun 12;56(25):7227-7232. doi: 10.1002/anie.201703155. Epub 2017 May 19. Angew Chem Int Ed Engl. 2017. PMID: 28523779 Free PMC article.
-
The isomerization of allylrhodium intermediates in the rhodium-catalyzed nucleophilic allylation of cyclic imines.Angew Chem Int Ed Engl. 2014 Oct 20;53(43):11605-10. doi: 10.1002/anie.201407233. Epub 2014 Sep 9. Angew Chem Int Ed Engl. 2014. PMID: 25205604 Free PMC article.
-
Remote Nucleophilic Allylation by Allylrhodium Chain Walking.Chemistry. 2018 Sep 12;24(51):13432-13436. doi: 10.1002/chem.201803574. Epub 2018 Aug 20. Chemistry. 2018. PMID: 30006953
-
Rhodium-Catalyzed (5 + 2) and (5 + 1) Cycloadditions Using 1,4-Enynes as Five-Carbon Building Blocks.Acc Chem Res. 2020 Jan 21;53(1):231-243. doi: 10.1021/acs.accounts.9b00477. Epub 2019 Dec 10. Acc Chem Res. 2020. PMID: 31820914 Free PMC article. Review.
Cited by
-
Base mediated aza-[2 + 1] annulation and regioselective aziridine ring-opening cascade: mild synthesis of functionalized β-amino ketones from cyclic N-sulfonyl aldimines and α-carbonyl sulfonium salts.RSC Adv. 2024 May 28;14(24):17178-17183. doi: 10.1039/d4ra02817a. eCollection 2024 May 22. RSC Adv. 2024. PMID: 38808243 Free PMC article.
-
Enantioselective Rhodium-Catalyzed Coupling of Arylboronic Acids, 1,3-Enynes, and Imines by Alkenyl-to-Allyl 1,4-Rhodium(I) Migration.Angew Chem Int Ed Engl. 2017 Dec 18;56(51):16352-16356. doi: 10.1002/anie.201709334. Epub 2017 Nov 24. Angew Chem Int Ed Engl. 2017. PMID: 28980437 Free PMC article.
-
Simultaneous Stereoinvertive and Stereoselective C(sp3)-C(sp3) Cross-Coupling of Boronic Esters and Allylic Carbonates.J Am Chem Soc. 2024 May 22;146(20):13719-13726. doi: 10.1021/jacs.4c03686. Epub 2024 May 9. J Am Chem Soc. 2024. PMID: 38721780 Free PMC article.
-
Selective Synthesis of Z-Silyl Enol Ethers via Ni-Catalyzed Remote Functionalization of Ketones.J Am Chem Soc. 2021 Jun 9;143(22):8375-8380. doi: 10.1021/jacs.1c01797. Epub 2021 May 25. J Am Chem Soc. 2021. PMID: 34033717 Free PMC article.
-
Arylative Intramolecular Allylation of Ketones with 1,3-Enynes Enabled by Catalytic Alkenyl-to-Allyl 1,4-Rhodium(I) Migration.Angew Chem Int Ed Engl. 2017 Jun 12;56(25):7227-7232. doi: 10.1002/anie.201703155. Epub 2017 May 19. Angew Chem Int Ed Engl. 2017. PMID: 28523779 Free PMC article.
References
-
- For chain walking polymerization, see:
-
- Guan Z., Cotts P. M., McCord E. F., McLain S. J., Science 1999, 283, 2059–2062; - PubMed
-
- Ittel S. D., Johnson L. K., Brookhart M., Chem. Rev. 2000, 100, 1169–1204; - PubMed
-
- Dong Z., Ye Z., Polym. Chem. 2012, 3, 286–301.
-
- For a review of catalytic alkene isomerization-hydroformylation reactions, see: Vilches-Herrera M., Domke L., Börner A., ACS Catal. 2014, 4, 1706–1724.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

