Phase Space Bottlenecks in Enzymatic Reactions
- PMID: 26756622
- PMCID: PMC4734068
- DOI: 10.1021/acs.jpcb.5b11157
Phase Space Bottlenecks in Enzymatic Reactions
Abstract
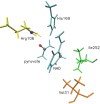
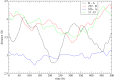
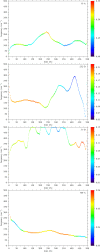
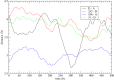
The definition of a transition state on an individual reactive trajectory is made via a committor analysis. In the past, the bottleneck definition has often been applied in configuration space. This is an approximation, and in order to expand this definition, we are revisiting an enzyme in which we had identified a fast subpicosecond motion that makes the reaction possible. First we used a time-series analysis method to identify the exact time when this motion initiates donor-acceptor compression. Then we modified the standard committor analysis of transition path sampling to identify events in phase space and found that there is a dividing surface in phase space significantly earlier than the configurationally defined transition-state crossing.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Garrett B.; Truhlar D. In Theory and Applications of Computational Chemistry: the first forty years; Dykstra C., Frenking G., Kim K., Scuseria G., Eds.; Elsevier: 2005.
-
- Bolhuis P.; Dellago C. Practical and conceptual path sampling issues. Eur. Phys. J.: Spec. Top. 2015, 224, 2409–2427. 10.1140/epjst/e2015-02419-6. - DOI
-
- Gertner B. J.; Bergsma J. P.; Wilson K. R.; lee S.; Hynes J. T. Nonadiabatic solvation model for SN2 reactions in polar solvents. J. Chem. Phys. 1987, 86, 1377.10.1063/1.452225. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

