Cytochrome P450 and Lipoxygenase Metabolites on Renal Function
- PMID: 26756638
- PMCID: PMC5965260
- DOI: 10.1002/cphy.c150009
Cytochrome P450 and Lipoxygenase Metabolites on Renal Function
Abstract
Arachidonic acid metabolites have a myriad of biological actions including effects on the kidney to alter renal hemodynamics and tubular transport processes. Cyclooxygenase metabolites are products of an arachidonic acid enzymatic pathway that has been extensively studied in regards to renal function. Two lesser-known enzymatic pathways of arachidonic acid metabolism are the lipoxygenase (LO) and cytochrome P450 (CYP) pathways. The importance of LO and CYP metabolites to renal hemodynamics and tubular transport processes is now being recognized. LO and CYP metabolites have actions to alter renal blood flow and glomerular filtration rate. Proximal and distal tubular sodium transport and fluid and electrolyte homeostasis are also significantly influenced by renal CYP and LO levels. Metabolites of the LO and CYP pathways also have renal actions that influence renal inflammation, proliferation, and apoptotic processes at vascular and epithelial cells. These renal LO and CYP pathway actions occur through generation of specific metabolites and cell-signaling mechanisms. Even though the renal physiological importance and actions for LO and CYP metabolites are readily apparent, major gaps remain in our understanding of these lipid mediators to renal function. Future studies will be needed to fill these major gaps regarding LO and CYP metabolites on renal function.
Copyright © 2015 John Wiley & Sons, Inc.
Figures
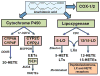
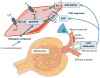

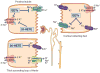

References
-
- Alonso-Galicia M, Drummond HA, Reddy KK, Falck JR, Roman RJ. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension. 1997;29:320–325. - PubMed
-
- Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol. 1999;277:F790–F796. - PubMed
-
- Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Roman RJ. Role of 20-Hydroxyeicosatetraeinoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regulatory, Integrative Comp Physiol. 2002;283:R60–R68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

