Prevalence of Submandibular Gland Synucleinopathy in Parkinson's Disease, Dementia with Lewy Bodies and other Lewy Body Disorders
- PMID: 26756744
- PMCID: PMC5498170
- DOI: 10.3233/JPD-150680
Prevalence of Submandibular Gland Synucleinopathy in Parkinson's Disease, Dementia with Lewy Bodies and other Lewy Body Disorders
Abstract
Background: Clinical misdiagnosis, particularly at early disease stages, is a roadblock to finding new therapies for Lewy body disorders. Biopsy of a peripheral site might provide improved diagnostic accuracy. Previously, we reported, from both autopsy and needle biopsy, a high prevalence of submandibular gland synucleinopathy in Parkinson's disease (PD). Here, we report on an extension of these studies to subjects with dementia with Lewy bodies (DLB) and other Lewy body disorders in 228 autopsied subjects from the Arizona Study of Aging and Neurodegenerative Disorders.
Objective: To provide an estimate of the prevalence of histological synucleinopathy in the submandibular glands of subjects with PD and other Lewy body disorders.
Methods: Submandibular gland sections from autopsied subjects were stained with an immunohistochemical method for α-synuclein phosphorylated at serine 129. Included were 146 cases with CNS Lewy-type synucleinopathy (LTS), composed of 46 PD, 28 DLB, 14 incidental Lewy body disease (ILBD), 33 Alzheimer's disease with Lewy bodies (ADLB) and 2 with progressive supranuclear palsy and Lewy bodies (PSPLB). Control subjects included 79 normal elderly, 15 AD, 12 PSP, 2 conticobasal degeneration (CBD) and 2 multiple system atrophy (MSA).
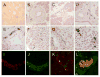
Results: Submandibular gland LTS was found in 42/47 (89%) of the PD subjects, 20/28 (71%) DLB, 4/33 (12%) ADLB and 1/9 (11%) ILBD subjects but none of the 110 control subjects.
Conclusions: These results provide support for further clinical trials of in vivo submandibular gland diagnostic biopsy for PD and DLB. An accurate peripheral biopsy diagnosis would assist subject selection for clinical trials and could also be used to verify other biomarkers.
Keywords: Biopsy; biomarker; clinical trial; diagnosis; pathology; therapy.
Figures
References
-
- Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology. 2001;57:1497–1499. - PubMed
-
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. - PubMed
-
- Cornford ME, Chang L, Miller BL. The neuropathology of parkinsonism: an overview. Brain Cogn. 1995;28:321–341. - PubMed
-
- Litvan I, MacIntyre A, Goetz CG, Wenning GK, Jellinger K, Verny M, Bartko JJ, Jankovic J, McKee A, Brandel JP, Chaudhuri KR, Lai EC, D’Olhaberriague L, Pearce RK, Agid Y. Accuracy of the clinical diagnoses of Lewy body disease, Parkinson disease, and dementia with Lewy bodies: a clinicopathologic study. Arch Neurol. 1998;55:969–978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

