Lidocaine Inhibits HCN Currents in Rat Spinal Substantia Gelatinosa Neurons
- PMID: 26756913
- PMCID: PMC4791316
- DOI: 10.1213/ANE.0000000000001140
Lidocaine Inhibits HCN Currents in Rat Spinal Substantia Gelatinosa Neurons
Abstract
Background: Lidocaine, which blocks voltage-gated sodium channels, is widely used in surgical anesthesia and pain management. Recently, it has been proposed that the hyperpolarization-activated cyclic nucleotide (HCN) channel is one of the other novel targets of lidocaine. Substantia gelatinosa in the spinal dorsal horn, which plays key roles in modulating nociceptive information from primary afferents, comprises heterogeneous interneurons that can be electrophysiologically categorized by firing pattern. Our previous study demonstrated that a substantial proportion of substantia gelatinosa neurons reveal the presence of HCN current (Ih); however, the roles of lidocaine and HCN channel expression in different types of substantia gelatinosa neurons remain unclear.
Methods: By using the whole-cell patch-clamp technique, we investigated the effect of lidocaine on Ih in rat substantia gelatinosa neurons of acute dissociated spinal cord slices.
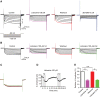
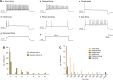
Results: We found that lidocaine rapidly decreased the peak Ih amplitude with an IC50 of 80 μM. The inhibition rate on Ih was not significantly different with a second application of lidocaine in the same neuron. Tetrodotoxin, a sodium channel blocker, did not affect lidocaine's effect on Ih. In addition, lidocaine shifted the half-activation potential of Ih from -109.7 to -114.9 mV and slowed activation. Moreover, the reversal potential of Ih was shifted by -7.5 mV by lidocaine. In the current clamp, lidocaine decreased the resting membrane potential, increased membrane resistance, delayed rebound depolarization latency, and reduced the rebound spike frequency. We further found that approximately 58% of substantia gelatinosa neurons examined expressed Ih, in which most of them were tonically firing.
Conclusions: Our studies demonstrate that lidocaine strongly inhibits Ih in a reversible and concentration-dependent manner in substantia gelatinosa neurons, independent of tetrodotoxin-sensitive sodium channels. Thus, our study provides new insight into the mechanism underlying the central analgesic effect of the systemic administration of lidocaine.
Conflict of interest statement
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81000480 and 81260175) and Natural Science Foundation of Jiangxi, China (No. 20151BAB204022).
The authors declare no conflicts of interest.
Figures





Similar articles
-
Minocycline inhibits hyperpolarization-activated currents in rat substantia gelatinosa neurons.Neuropharmacology. 2015 Aug;95:110-20. doi: 10.1016/j.neuropharm.2015.03.001. Epub 2015 Mar 14. Neuropharmacology. 2015. PMID: 25777286
-
Contribution of presynaptic HCN channels to excitatory inputs of spinal substantia gelatinosa neurons.Neuroscience. 2017 Sep 1;358:146-157. doi: 10.1016/j.neuroscience.2017.06.046. Epub 2017 Jul 1. Neuroscience. 2017. PMID: 28673721
-
[Rebound depolarization of substantia gelatinosa neurons and its modulatory mechanisms in rat spinal dorsal horn].Nan Fang Yi Ke Da Xue Xue Bao. 2016 Feb 20;37(2):204-209. doi: 10.3969/j.issn.1673-4254.2017.02.10. Nan Fang Yi Ke Da Xue Xue Bao. 2016. PMID: 28219864 Free PMC article. Chinese.
-
HCN Channels: New Therapeutic Targets for Pain Treatment.Molecules. 2018 Aug 21;23(9):2094. doi: 10.3390/molecules23092094. Molecules. 2018. PMID: 30134541 Free PMC article. Review.
-
Ih from synapses to networks: HCN channel functions and modulation in neurons.Prog Biophys Mol Biol. 2021 Nov;166:119-132. doi: 10.1016/j.pbiomolbio.2021.06.002. Epub 2021 Jun 25. Prog Biophys Mol Biol. 2021. PMID: 34181891 Free PMC article. Review.
Cited by
-
An electrophysiologist's guide to dorsal horn excitability and pain.Front Cell Neurosci. 2025 Apr 2;19:1548252. doi: 10.3389/fncel.2025.1548252. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40241846 Free PMC article. Review.
-
The role of lidocaine in cancer progression and patient survival.Pharmacol Ther. 2024 Jul;259:108654. doi: 10.1016/j.pharmthera.2024.108654. Epub 2024 May 1. Pharmacol Ther. 2024. PMID: 38701900 Free PMC article. Review.
-
Cell-Type Specific Distribution of T-Type Calcium Currents in Lamina II Neurons of the Rat Spinal Cord.Front Cell Neurosci. 2018 Oct 17;12:370. doi: 10.3389/fncel.2018.00370. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30386213 Free PMC article.
-
Readiness of nociceptor cell bodies to generate spontaneous activity results from background activity of diverse ion channels and high input resistance.Pain. 2024 Apr 1;165(4):893-907. doi: 10.1097/j.pain.0000000000003091. Epub 2023 Oct 20. Pain. 2024. PMID: 37862056 Free PMC article.
-
Topical Treatments and Their Molecular/Cellular Mechanisms in Patients with Peripheral Neuropathic Pain-Narrative Review.Pharmaceutics. 2021 Mar 26;13(4):450. doi: 10.3390/pharmaceutics13040450. Pharmaceutics. 2021. PMID: 33810493 Free PMC article. Review.
References
-
- Smith LJ, Bentley E, Shih A, Miller PE. Systemic lidocaine infusion as an analgesic for intraocular surgery in dogs: a pilot study. Vet Anaesth Analg. 2004;31:53–63. - PubMed
-
- McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs. 2010;70:1149–63. - PubMed
-
- Vigneault L, Turgeon AF, Côté D, Lauzier F, Zarychanski R, Moore L, McIntyre LA, Nicole PC, Fergusson DA. Perioperative intravenous lidocaine infusion for postoperative pain control: a meta-analysis of randomized controlled trials. Can J Anaesth. 2011;58:22–37. - PubMed
-
- Butterworth JF, Strichartz GR. Molecular mechanisms of local anesthesia: a review. Anesthesiology. 1990;72:711–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

