Towards comprehensive cardiac repair and regeneration after myocardial infarction: Aspects to consider and proteins to deliver
- PMID: 26757257
- PMCID: PMC4872516
- DOI: 10.1016/j.biomaterials.2015.12.025
Towards comprehensive cardiac repair and regeneration after myocardial infarction: Aspects to consider and proteins to deliver
Abstract
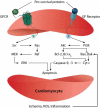
Ischemic heart disease is a leading cause of death worldwide. After the onset of myocardial infarction, many pathological changes take place and progress the disease towards heart failure. Pathologies such as ischemia, inflammation, cardiomyocyte death, ventricular remodeling and dilation, and interstitial fibrosis, develop and involve the signaling of many proteins. Proteins can play important roles in limiting or countering pathological changes after infarction. However, they typically have short half-lives in vivo in their free form and can benefit from the advantages offered by controlled release systems to overcome their challenges. The controlled delivery of an optimal combination of proteins per their physiologic spatiotemporal cues to the infarcted myocardium holds great potential to repair and regenerate the heart. The effectiveness of therapeutic interventions depends on the elucidation of the molecular mechanisms of the cargo proteins and the spatiotemporal control of their release. It is likely that multiple proteins will provide a more comprehensive and functional recovery of the heart in a controlled release strategy.
Keywords: Biomaterials; Controlled release; Delivery systems; Extracellular matrix; Myocardial infarction; Protein therapy.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures









References
-
- Mendis S, Puska P, Norrving B, World Health Organization., World Heart Federation., World Stroke Organization . Global atlas on cardiovascular disease prevention and control. World Health Organization in collaboration with the World Heart Federation and the World Stroke Organization; Geneva: 2011.
-
- Choi D, Hwang KC, Lee KY, Kim YH. Ischemic heart diseases: current treatments and future. J Control Release. 2009;140:194–202. - PubMed
-
- White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

