Cathepsin L inactivation leads to multimodal inhibition of prostate cancer cell dissemination in a preclinical bone metastasis model
- PMID: 26757413
- PMCID: PMC4805507
- DOI: 10.1002/ijc.29992
Cathepsin L inactivation leads to multimodal inhibition of prostate cancer cell dissemination in a preclinical bone metastasis model
Abstract
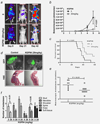
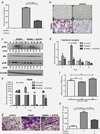
It is estimated that approximately 90% of patients with advanced prostate cancer develop bone metastases; an occurrence that results in a substantial reduction in the quality of life and a drastic worsening of prognosis. The development of novel therapeutic strategies that impair the metastatic process and associated skeletal adversities is therefore critical to improving prostate cancer patient survival. Recognition of the importance of Cathepsin L (CTSL) to metastatic dissemination of cancer cells has led to the development of several CTSL inhibition strategies. The present investigation employed intra-cardiac injection of human PC-3ML prostate cancer cells into nude mice to examine tumor cell dissemination in a preclinical bone metastasis model. CTSL knockdown confirmed the validity of targeting this protease and subsequent intervention studies with the small molecule CTSL inhibitor KGP94 resulted in a significant reduction in metastatic tumor burden in the bone and an improvement in overall survival. CTSL inhibition by KGP94 also led to a significant impairment of tumor initiated angiogenesis. Furthermore, KGP94 treatment decreased osteoclast formation and bone resorptive function, thus, perturbing the reciprocal interactions between tumor cells and osteoclasts within the bone microenvironment which typically result in bone loss and aggressive growth of metastases. These functional effects were accompanied by a significant downregulation of NFκB signaling activity and expression of osteoclastogenesis related NFκB target genes. Collectively, these data indicate that the CTSL inhibitor KGP94 has the potential to alleviate metastatic disease progression and associated skeletal morbidities and hence may have utility in the treatment of advanced prostate cancer patients.
Keywords: KGP94; bone resorption; cathepsin L; metastasis; prostate cancer.
© 2016 UICC.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures




Similar articles
-
Cathepsin L in tumor angiogenesis and its therapeutic intervention by the small molecule inhibitor KGP94.Clin Exp Metastasis. 2016 Jun;33(5):461-73. doi: 10.1007/s10585-016-9790-1. Epub 2016 Apr 7. Clin Exp Metastasis. 2016. PMID: 27055649 Free PMC article.
-
Cathepsin L inhibition by the small molecule KGP94 suppresses tumor microenvironment enhanced metastasis associated cell functions of prostate and breast cancer cells.Clin Exp Metastasis. 2013 Oct;30(7):891-902. doi: 10.1007/s10585-013-9590-9. Epub 2013 Jun 9. Clin Exp Metastasis. 2013. PMID: 23748470 Free PMC article.
-
The MET/Vascular Endothelial Growth Factor Receptor (VEGFR)-targeted Tyrosine Kinase Inhibitor Also Attenuates FMS-dependent Osteoclast Differentiation and Bone Destruction Induced by Prostate Cancer.J Biol Chem. 2016 Sep 30;291(40):20891-20899. doi: 10.1074/jbc.M116.727875. Epub 2016 Aug 18. J Biol Chem. 2016. PMID: 27539855 Free PMC article.
-
Cathepsin L targeting in cancer treatment.Pharmacol Ther. 2015 Nov;155:105-16. doi: 10.1016/j.pharmthera.2015.08.007. Epub 2015 Aug 20. Pharmacol Ther. 2015. PMID: 26299995 Free PMC article. Review.
-
Cathepsin K inhibitors as treatment of bone metastasis.Curr Opin Support Palliat Care. 2008 Sep;2(3):218-22. doi: 10.1097/SPC.0b013e32830baea9. Curr Opin Support Palliat Care. 2008. PMID: 18685424 Review.
Cited by
-
Bone-targeted therapies to reduce skeletal morbidity in prostate cancer.Asian J Androl. 2018 May-Jun;20(3):215-220. doi: 10.4103/aja.aja_12_18. Asian J Androl. 2018. PMID: 29553053 Free PMC article. Review.
-
Stromal cells in breast cancer as a potential therapeutic target.Oncotarget. 2018 May 4;9(34):23761-23779. doi: 10.18632/oncotarget.25245. eCollection 2018 May 4. Oncotarget. 2018. PMID: 29805773 Free PMC article. Review.
-
Overexpression of Cathepsin L is associated with chemoresistance and invasion of epithelial ovarian cancer.Oncotarget. 2016 Jul 19;7(29):45995-46001. doi: 10.18632/oncotarget.10276. Oncotarget. 2016. PMID: 27351223 Free PMC article.
-
Methyltransferase-like 3 promotes cervical cancer metastasis by enhancing cathepsin L mRNA stability in an N6-methyladenosine-dependent manner.Cancer Sci. 2023 Mar;114(3):837-854. doi: 10.1111/cas.15658. Epub 2023 Jan 2. Cancer Sci. 2023. PMID: 36382580 Free PMC article.
-
The Potential of Extracellular Matrix- and Integrin Adhesion Complex-Related Molecules for Prostate Cancer Biomarker Discovery.Biomedicines. 2023 Dec 28;12(1):79. doi: 10.3390/biomedicines12010079. Biomedicines. 2023. PMID: 38255186 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J Clin. 2015;65:5–29. - PubMed
-
- Norgaard M, Jensen AO, Jacobsen JB, et al. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007) J Urol. 2010;184:162–7. - PubMed
-
- Soloway MS, Hardeman SW, Hickey D, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;61:195–202. - PubMed
-
- Carlin BI, Andriole GL. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer. 2000;88:2989–94. - PubMed
-
- Palermo C, Joyce JA. Cysteine cathepsin proteases as pharmacological targets in cancer. Trends Pharmacol Sci. 2008;29:22–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

