CAPG and GIPC1: Breast Cancer Biomarkers for Bone Metastasis Development and Treatment
- PMID: 26757732
- PMCID: PMC4808632
- DOI: 10.1093/jnci/djv360
CAPG and GIPC1: Breast Cancer Biomarkers for Bone Metastasis Development and Treatment
Erratum in
-
Erratum.J Natl Cancer Inst. 2016 Feb 10;108(3):djw017. doi: 10.1093/jnci/djw017. Print 2016 Mar. J Natl Cancer Inst. 2016. PMID: 26868856 Free PMC article. No abstract available.
Abstract
Background: Bone is the predominant site of metastasis from breast cancer, and recent trials have demonstrated that adjuvant bisphosphonate therapy can reduce bone metastasis development and improve survival. There is an unmet need for prognostic and predictive biomarkers so that therapy can be appropriately targeted.
Methods: Potential biomarkers for bone metastasis were identified using proteomic comparison of bone-metastatic, lung-metastatic, and nonmetastatic variants of human breast cancer MDA-MB-231 cells. Clinical validation was performed using immunohistochemical staining of tumor tissue microarrays from patients in a large randomized trial of adjuvant zoledronic acid (zoledronate) (AZURE-ISRCTN79831382). We used Cox proportional hazards regression, the Kaplan-Meier estimate of the survival function, and the log-rank test to investigate associations between protein expression, clinical variables, and time to distant recurrence events. All statistical tests were two-sided.
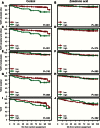
Results: Two novel biomarker candidates, macrophage-capping protein (CAPG) and PDZ domain-containing protein GIPC1 (GIPC1), were identified for clinical validation. Cox regression analysis of AZURE training and validation sets showed that control patients (no zoledronate) were more likely to develop first distant recurrence in bone (hazard ratio [HR] = 4.5, 95% confidence interval [CI] = 2.1 to 9.8, P < .001) and die (HR for overall survival = 1.8, 95% CI = 1.01 to 3.24, P = .045) if both proteins were highly expressed in the primary tumor. In patients with high expression of both proteins, zoledronate had a substantial effect, leading to 10-fold hazard ratio reduction (compared with control) for first distant recurrence in bone (P = .008).
Conclusions: The composite biomarker, CAPG and GIPC1 in primary breast tumors, predicted disease outcomes and benefit from zoledronate and may facilitate patient selection for adjuvant bisphosphonate treatment.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures




References
-
- Coleman RE, Marshall H, Cameron D, et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365(15):1396–1405. - PubMed
-
- Gnant M, Mlineritsch B, Stoeger H, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomized trial. Lancet Oncol. 2011;12 (7):631–641. - PubMed
-
- Coleman RE, Cameron D, Dodwell D, et al. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomized open-label phase 3 trial. Lancet Oncol. 2014;15(9):997–1006. - PubMed
-
- Coleman R, Gnant M, Paterson A, et al. Effects of bisphosphonate treatment on recurrence and cause-specific mortality in women early breast cancer: A meta-analysis of individual patient data from randomized trials. San Antonio Breast Cancer Symposium, San Antonio, TX, USA: December 10–14, 2013. Abstract S4-07.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

