Methylation-dependent regulation of HIF-1α stability restricts retinal and tumour angiogenesis
- PMID: 26757928
- PMCID: PMC4735525
- DOI: 10.1038/ncomms10347
Methylation-dependent regulation of HIF-1α stability restricts retinal and tumour angiogenesis
Abstract
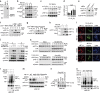
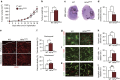
Hypoxia-inducible factor-1α (HIF-1α) mediates hypoxic responses and regulates gene expression involved in angiogenesis, invasion and metabolism. Among the various HIF-1α posttranslational modifications, HIF-1α methylation and its physiological role have not yet been elucidated. Here we show that HIF-1α is methylated by SET7/9 methyltransferase, and that lysine-specific demethylase 1 reverses its methylation. The functional consequence of HIF-1α methylation is the modulation of HIF-1α stability primarily in the nucleus, independent of its proline hydroxylation, during long-term hypoxic and normoxic conditions. Knock-in mice bearing a methylation-defective Hif1a(KA/KA) allele exhibit enhanced retinal angiogenesis and tumour vascularization via HIF-1α stabilization. Importantly, S28Y and R30Q mutations of HIF-1α, found in human cancers, are involved in the altered HIF-1α stability. Together, these results demonstrate a role for HIF-1α methylation in regulating protein stability, thereby modulating biological output including retinal and tumour angiogenesis, with therapeutic implications in human cancer.
Figures





References
-
- Cassavaugh J. & Lounsbury K. M. Hypoxia-mediated biological control. J. Cell Biochem. 112, 735–744 (2011). - PubMed
-
- Semenza G. L. Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 365, 537–547 (2011). - PubMed
-
- Ho T. K. et al.. Increased endogenous angiogenic response and hypoxia-inducible factor-1alpha in human critical limb ischemia. J. Vasc. Surg. 43, 125–133 (2006). - PubMed
-
- Bosch-Marce M. et al.. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ. Res. 101, 1310–1318 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

