A novel, clinically relevant use of a piglet model to study the effects of anesthetics on the developing brain
- PMID: 26757938
- PMCID: PMC4710621
- DOI: 10.1186/s40169-015-0079-9
A novel, clinically relevant use of a piglet model to study the effects of anesthetics on the developing brain
Abstract
Background: Anesthesia-induced neurotoxicity research in the developing brain must rely upon an unimpeachable animal model and a standardized treatment approach. In this manner, identification of mechanisms of action may be undertaken. The goal of this study was to develop a novel, clinically relevant, translational way to use a piglet model to investigate anesthesia effects on the developing brain.
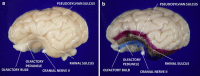
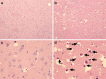
Methods: 29 newborn piglets were assigned to either: (1) control (no intervention, n = 10); (2) lipopolysaccharide (LPS; positive inflammatory control, n = 9); or (3) isoflurane anesthesia (n = 10). Positive inflammatory control animals were given 100 mcg/kg LPS from Escherichia coli intraperitoneally (IP) on the same day as those receiving isoflurane. Isoflurane was administered for 3 h while care was taken to ensure human perioperative conditions. To establish a clinical scenario, each animal was intubated and monitored with pulse oximetry, invasive and non-invasive blood pressure, electrocardiogram, temperature, end-tidal CO2, anesthetic concentration, and iSTAT blood analysis. All animals were sacrificed after 48 h using transcardiac perfusion of ice-cold, heparinized phosphate buffered saline (PBS) followed by 4 % paraformaldehyde (PFA). Brains were collected and histopathological analysis focused on the entorhinal cortex looking for degenerative changes due to its critical role in learning and memory. Reliable identification of entorhinal cortex was achieved by using colored ink on the surface of the brains, which was then cross-referenced with microscopic anatomy. Hematoxylin & eosin-stained high-power fields was used to quantify cells. ImageJ™ (National Institutes of Health, Bethesda, MD, USA) was used to count absolute number of progenitor glial cells (PGC) and number of PGCs per cluster. Immunohistochemistry was also utilized to ensure positive identification of cellular structures.
Results: Histopathological sections of 28 brains were analyzed. One animal in the LPS group died shortly after administration, presumably from inadvertent intravascular injection. There was an acute basal ganglia ischemic infarct in one isoflurane-treated animal. A large number of small, round nucleated cells were seen throughout layer II of the entorhinal cortex in all animals. These cells were identified as PGCs using immunohistochemistry and light microscopy. Although there was no difference in the absolute number of PGCs between the groups, animals given isoflurane or LPS demonstrated a significant increase in cells forming 'clusters' in the entorhinal cortex. An apparent change in the pattern of doublecortin labeling also suggests changes in neuronal precursors and undifferentiated neurons.
Conclusions: This study represents the first novel use of a clinically relevant neonatal piglet model to study anesthesia effects on the developing brain. LPS induces neuroinflammation, and this is a potential mechanism for LPS and perhaps isoflurane in causing a change in progenitor cell distribution. We postulate that the isoflurane-induced change in glial progenitor cell distribution could have important implications for cell differentiation, maturation and neural circuit behavior in the rapidly developing brain.
Keywords: Anesthesia; Hippocampus; Isoflurane; Neurocognitive outcome; Neurodevelopment; Neuroinflammation; Neurotoxicity; Piglets.
Figures







Similar articles
-
The effect of prolonged anesthesia with isoflurane, propofol, dexmedetomidine, or ketamine on neural cell proliferation in the adult rat.Anesth Analg. 2008 Jun;106(6):1772-7. doi: 10.1213/ane.0b013e31816f2004. Anesth Analg. 2008. PMID: 18499608
-
Neuropathology in intrauterine growth restricted newborn piglets is associated with glial activation and proinflammatory status in the brain.J Neuroinflammation. 2019 Jan 8;16(1):5. doi: 10.1186/s12974-018-1392-1. J Neuroinflammation. 2019. PMID: 30621715 Free PMC article.
-
Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain.Anesthesiology. 2010 Apr;112(4):834-41. doi: 10.1097/ALN.0b013e3181d049cd. Anesthesiology. 2010. PMID: 20234312 Free PMC article.
-
Beyond anesthetic properties: the effects of isoflurane on brain cell death, neurogenesis, and long-term neurocognitive function.Anesth Analg. 2010 Feb;110(2):431-7. Anesth Analg. 2010. PMID: 25508825 Review.
-
Beyond anesthetic properties: the effects of isoflurane on brain cell death, neurogenesis, and long-term neurocognitive function.Anesth Analg. 2010 Feb;110(2):431-7. doi: 10.1213/ANE.0b013e3181af8015. Anesth Analg. 2010. PMID: 19917621 Review.
Cited by
-
Selective induction of IL-1β after a brief isoflurane anesthetic in children undergoing MRI examination.J Anesth. 2017 Apr;31(2):219-224. doi: 10.1007/s00540-016-2294-y. Epub 2017 Jan 3. J Anesth. 2017. PMID: 28050702 Clinical Trial.
-
Early-Life Nutrition and Neurodevelopment: Use of the Piglet as a Translational Model.Adv Nutr. 2017 Jan 17;8(1):92-104. doi: 10.3945/an.116.013243. Print 2017 Jan. Adv Nutr. 2017. PMID: 28096130 Free PMC article. Review.
-
Anesthetics inhibit phosphorylation of the ribosomal protein S6 in mouse cultured cortical cells and developing brain.Front Aging Neurosci. 2023 May 16;15:1060186. doi: 10.3389/fnagi.2023.1060186. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37261265 Free PMC article.
-
Standards for preclinical research and publications in developmental anaesthetic neurotoxicity: expert opinion statement from the SmartTots preclinical working group.Br J Anaesth. 2020 May;124(5):585-593. doi: 10.1016/j.bja.2020.01.011. Epub 2020 Mar 4. Br J Anaesth. 2020. PMID: 32145876 Free PMC article. Review.
-
Adaptation of Microelectrode Array Technology for the Study of Anesthesia-induced Neurotoxicity in the Intact Piglet Brain.J Vis Exp. 2018 May 12;(135):57391. doi: 10.3791/57391. J Vis Exp. 2018. PMID: 29806825 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
