Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion
- PMID: 26758544
- PMCID: PMC4741299
- DOI: 10.15252/embj.201592705
Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion
Abstract
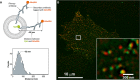
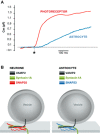
Astrocytes are housekeepers of the central nervous system (CNS) and are important for CNS development, homeostasis and defence. They communicate with neurones and other glial cells through the release of signalling molecules. Astrocytes secrete a wide array of classic neurotransmitters, neuromodulators and hormones, as well as metabolic, trophic and plastic factors, all of which contribute to the gliocrine system. The release of neuroactive substances from astrocytes occurs through several distinct pathways that include diffusion through plasmalemmal channels, translocation by multiple transporters and regulated exocytosis. As in other eukaryotic cells, exocytotic secretion from astrocytes involves divergent secretory organelles (synaptic-like microvesicles, dense-core vesicles, lysosomes, exosomes and ectosomes), which differ in size, origin, cargo, membrane composition, dynamics and functions. In this review, we summarize the features and functions of secretory organelles in astrocytes. We focus on the biogenesis and trafficking of secretory organelles and on the regulation of the exocytotic secretory system in the context of healthy and diseased astrocytes.
Keywords: SNARE proteins; astrocytes; exocytosis; secretion; secretory vesicles.
© 2016 The Authors.
Figures


References
-
- Andrews NW, Chakrabarti S (2005) There's more to life than neurotransmission: the regulation of exocytosis by synaptotagmin VII. Trends Cell Biol 15: 626–631 - PubMed
-
- Arcienega II, Brunet JF, Bloch J, Badaut J (2010) Cell locations for AQP1, AQP4 and 9 in the non‐human primate brain. Neuroscience 167: 1103–1114 - PubMed
-
- Arima H, Yamamoto N, Sobue K, Umenishi F, Tada T, Katsuya H, Asai K (2003) Hyperosmolar mannitol simulates expression of aquaporins 4 and 9 through a p38 mitogen‐activated protein kinase‐dependent pathway in rat astrocytes. J Biol Chem 278: 44525–44534 - PubMed
-
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G (2012) Syndecan‐syntenin‐ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14: 677–685 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

