In Vivo MRI Mapping of Brain Iron Deposition across the Adult Lifespan
- PMID: 26758829
- PMCID: PMC4710766
- DOI: 10.1523/JNEUROSCI.1907-15.2016
In Vivo MRI Mapping of Brain Iron Deposition across the Adult Lifespan
Abstract
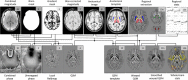
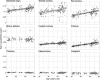
Disruption of iron homeostasis as a consequence of aging is thought to cause iron levels to increase, potentially promoting oxidative cellular damage. Therefore, understanding how this process evolves through the lifespan could offer insights into both the aging process and the development of aging-related neurodegenerative brain diseases. This work aimed to map, in vivo for the first time with an unbiased whole-brain approach, age-related iron changes using quantitative susceptibility mapping (QSM)--a new postprocessed MRI contrast mechanism. To this end, a full QSM standardization routine was devised and a cohort of N = 116 healthy adults (20-79 years of age) was studied. The whole-brain and ROI analyses confirmed that the propensity of brain cells to accumulate excessive iron as a function of aging largely depends on their exact anatomical location. Whereas only patchy signs of iron scavenging were observed in white matter, strong, bilateral, and confluent QSM-age associations were identified in several deep-brain nuclei--chiefly the striatum and midbrain-and across motor, premotor, posterior insular, superior prefrontal, and cerebellar cortices. The validity of QSM as a suitable in vivo imaging technique with which to monitor iron dysregulation in the human brain was demonstrated by confirming age-related increases in several subcortical nuclei that are known to accumulate iron with age. The study indicated that, in addition to these structures, there is a predilection for iron accumulation in the frontal lobes, which when combined with the subcortical findings, suggests that iron accumulation with age predominantly affects brain regions concerned with motor/output functions.
Significance statement: This study used a whole--brain imaging approach known as quantitative susceptibility mapping (QSM) to provide a novel insight into iron accumulation in the brain across the adult lifespan. Validity of the method was demonstrated by showing concordance with ROI analysis and prior knowledge of iron accumulation in subcortical nuclei. We discovered that, beyond these regions, there is extensive involvement of the frontal lobes that has been missed by past ROI analyses. Broadly speaking, therefore, the motor system selectively accumulates iron with age. The results offer insights into the aging process, but also offer a new approach to studying the role of iron dysregulation in the evolution of age-related neurodegenerative diseases.
Keywords: aging; brain iron; magnetic susceptibility; neurodegenerative diseases.
Copyright © 2016 Acosta-Cabronero et al.
Figures



References
-
- Andrasi E, Pali N, Molnar Z, Kosel S. Brain aluminum, magnesium and phosphorus contents of control and Alzheimer-diseased patients. J Alzheimers Dis. 2005;7:273–284. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
