Isolated P/Q Calcium Channel Deletion in Layer VI Corticothalamic Neurons Generates Absence Epilepsy
- PMID: 26758833
- PMCID: PMC4710767
- DOI: 10.1523/JNEUROSCI.2555-15.2016
Isolated P/Q Calcium Channel Deletion in Layer VI Corticothalamic Neurons Generates Absence Epilepsy
Abstract
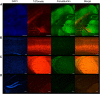
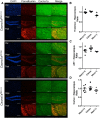
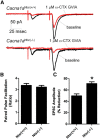
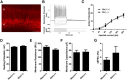
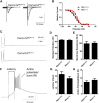
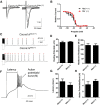
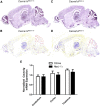
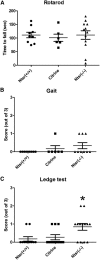
Generalized spike-wave seizures involving abnormal synchronization of cortical and underlying thalamic circuitry represent a major category of childhood epilepsy. Inborn errors of Cacna1a, the P/Q-type voltage-gated calcium channel α subunit gene, expressed throughout the brain destabilize corticothalamic rhythmicity and produce this phenotype. To determine the minimal cellular lesion required for this network disturbance, we used neurotensin receptor 1 (Ntsr1) cre-driver mice to ablate floxed Cacna1a in layer VI pyramidal neurons, which supply the sole descending cortical synaptic input to thalamocortical relay cells and reticular interneurons and activate intrathalamic circuits. Targeted Cacna1a ablation in layer VI cells resulted in mice that display a robust spontaneous spike-wave absence seizure phenotype accompanied by behavioral arrest and inhibited by ethosuximide. To verify the selectivity of the molecular lesion, we determined that P/Q subunit proteins were reduced in corticothalamic relay neuron terminal zones, and confirmed that P/Q-mediated glutamate release was reduced at these synapses. Spike-triggered exocytosis was preserved by N-type calcium channel rescue, demonstrating that evoked release at layer VI terminals relies on both P/Q and N-type channels. Whereas intrinsic excitability of the P/Q channel depleted layer VI neurons was unaltered, T-type calcium currents in the postsynaptic thalamic relay and reticular cells were dramatically elevated, favoring rebound bursting and seizure generation. We find that an early P/Q-type release defect, limited to synapses of a single cell-type within the thalamocortical circuit, is sufficient to remodel synchronized firing behavior and produce a stable generalized epilepsy phenotype.
Significance statement: This study dissects a critical component of the corticothalamic circuit in spike-wave epilepsy and identifies the developmental importance of P/Q-type calcium channel-mediated presynaptic glutamate release at layer VI pyramidal neuron terminals. Genetic ablation of Cacna1a in layer VI neurons produced synchronous spike-wave discharges in the cortex and thalamus that were inhibited by ethosuximide. These mice also displayed N-type calcium channel compensation at descending thalamic synapses, and consistent with other spike-wave models increased low-threshold T-type calcium currents within postsynaptic thalamic relay and reticular neurons. These results demonstrate, for the first time, that preventing the developmental homeostatic switch from loose to tightly coupled synaptic release at a single class of deep layer cortical excitatory output neurons results in generalized spike-wave epilepsy.
Keywords: Cacna1a; T-type; mouse; plasticity; spike-wave; tottering.
Copyright © 2016 the authors 0270-6474/16/360405-14$15.00/0.
Figures

References
-
- Berger H. Über das elektrenkephalogramm des menschen. Archiv Psychiatri Nervenkrankheit. 1933;98:231–254. doi: 10.1007/BF01814645. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
