Seizure-Induced Sympathoexcitation Is Caused by Activation of Glutamatergic Receptors in RVLM That Also Causes Proarrhythmogenic Changes Mediated by PACAP and Microglia in Rats
- PMID: 26758841
- PMCID: PMC6602029
- DOI: 10.1523/JNEUROSCI.2584-15.2016
Seizure-Induced Sympathoexcitation Is Caused by Activation of Glutamatergic Receptors in RVLM That Also Causes Proarrhythmogenic Changes Mediated by PACAP and Microglia in Rats
Abstract
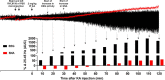
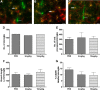
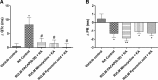
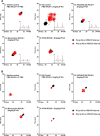
Cardiovascular autonomic dysfunction in seizure is a major cause of sudden unexpected death in epilepsy. The catecholaminergic neurons in the rostral ventrolateral medulla (RVLM) maintain sympathetic vasomotor tone and blood pressure through their direct excitatory projections to the intermediolateral (IML) cell column. Glutamate, the principal excitatory neurotransmitter in brain, is increased in seizures. Pituitary adenylate cyclase activating polypeptide (PACAP) is an excitatory neuropeptide with neuroprotective properties, whereas microglia are key players in inflammatory responses in CNS. We investigated the roles of glutamate, PACAP, and microglia on RVLM catecholaminergic neurons during the cardiovascular responses to 2 mg/kg kainic acid (KA)-induced seizures in urethane anesthetized, male Sprague Dawley rats. Microinjection of the glutamate antagonist, kynurenic acid (50 nl; 100 mM) into RVLM, blocked the seizure-induced 43.2 ± 12.6% sympathoexcitation (p ≤ 0.05), and abolished the pressor responses, tachycardia, and QT interval prolongation. PACAP or microglia antagonists (50 nl) (PACAP(6-38), 15 pmol; minocycline 10 mg/ml) microinjected bilaterally into RVLM had no effect on seizure-induced sympathoexcitation, pressor responses, or tachycardia but abolished the prolongation of QT interval. The actions of PACAP or microglia on RVLM neurons do not cause sympathoexcitation, but they do elicit proarrhythmogenic changes. An immunohistochemical analysis in 2 and 10 mg/kg KA-induced seizure rats revealed that microglia surrounding catecholaminergic neurons are in a "surveillance" state with no change in the number of M2 microglia (anti-inflammatory). In conclusion, seizure-induced sympathoexcitation is caused by activation of glutamatergic receptors in RVLM that also cause proarrhythmogenic changes mediated by PACAP and microglia.
Significance statement: Sudden unexpected death in epilepsy is a major cause of death in epilepsy. Generally, seizures are accompanied by changes in brain function leading to uncontrolled nerve activity causing high blood pressure, rapid heart rate, and abnormal heart rhythm. Nevertheless, the brain chemicals causing these cardiovascular changes are unknown. Chemicals, such as glutamate and pituitary adenylate cyclase activating polypeptide, whose expression is increased after seizures, act on specific cardiovascular nuclei in the brain and influence the activity of the heart, and blood vessels. Microglia, which manage excitation in the brain, are commonly activated after seizure and produce pro- and/or anti-inflammatory factors. Hence, we aimed to determine the effects of blocking glutamate, pituitary adenylate cyclase activating polypeptide, and microglia in the RVLM and their contribution to cardiovascular autonomic dysfunction in seizure.
Keywords: PACAP; glutamate; microglia; rat; seizure; sympathetic.
Copyright © 2016 the authors 0270-6474/16/360507-12$15.00/0.
Figures




Similar articles
-
Inhibition of microglial activation with minocycline at the intrathecal level attenuates sympathoexcitatory and proarrhythmogenic changes in rats with chronic temporal lobe epilepsy.Neuroscience. 2017 May 14;350:23-38. doi: 10.1016/j.neuroscience.2017.03.012. Epub 2017 Mar 18. Neuroscience. 2017. PMID: 28323007
-
PACAP-(6-38) or kynurenate microinjections in the RVLM prevent the development of sympathetic long-term facilitation after acute intermittent hypoxia.Am J Physiol Heart Circ Physiol. 2018 Mar 1;314(3):H563-H572. doi: 10.1152/ajpheart.00596.2017. Epub 2017 Dec 6. Am J Physiol Heart Circ Physiol. 2018. PMID: 29212793
-
Antagonism of PACAP or microglia function worsens the cardiovascular consequences of kainic-acid-induced seizures in rats.J Neurosci. 2015 Feb 4;35(5):2191-9. doi: 10.1523/JNEUROSCI.4058-14.2015. J Neurosci. 2015. PMID: 25653374 Free PMC article.
-
Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone.Acta Physiol Scand. 2003 Mar;177(3):209-18. doi: 10.1046/j.1365-201X.2003.01070.x. Acta Physiol Scand. 2003. PMID: 12608991 Review.
-
Rostral ventrolateral medulla: an integrative site for muscle vasodilation during defense-alerting reactions.Cell Mol Neurobiol. 2003 Oct;23(4-5):579-95. doi: 10.1023/a:1025076130854. Cell Mol Neurobiol. 2003. PMID: 14514017 Free PMC article. Review.
Cited by
-
Imaging Single-Cell Ca2+ Dynamics of Brainstem Neurons and Glia in Freely Behaving Mice.Bio Protoc. 2024 Apr 20;14(8):e4973. doi: 10.21769/BioProtoc.4973. eCollection 2024 Apr 20. Bio Protoc. 2024. PMID: 38737784 Free PMC article.
-
Orexin A increases sympathetic nerve activity through promoting expression of proinflammatory cytokines in Sprague Dawley rats.Acta Physiol (Oxf). 2018 Feb;222(2):10.1111/apha.12963. doi: 10.1111/apha.12963. Epub 2017 Oct 23. Acta Physiol (Oxf). 2018. PMID: 28872777 Free PMC article.
-
Microbiota-gut-brain axis and ketogenic diet: how close are we to tackling epilepsy?Microbiome Res Rep. 2023 Aug 29;2(4):32. doi: 10.20517/mrr.2023.24. eCollection 2023. Microbiome Res Rep. 2023. PMID: 38045924 Free PMC article. Review.
-
Neural correlate of reduced respiratory chemosensitivity during chronic epilepsy.Front Cell Neurosci. 2023 Dec 20;17:1288600. doi: 10.3389/fncel.2023.1288600. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38193031 Free PMC article.
-
Suppression of phrenic nerve activity as a potential predictor of imminent sudden unexpected death in epilepsy (SUDEP).Neuropharmacology. 2021 Feb 15;184:108405. doi: 10.1016/j.neuropharm.2020.108405. Epub 2020 Nov 16. Neuropharmacology. 2021. PMID: 33212114 Free PMC article.
References
-
- Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
