Decentralized Multisensory Information Integration in Neural Systems
- PMID: 26758843
- PMCID: PMC4710773
- DOI: 10.1523/JNEUROSCI.0578-15.2016
Decentralized Multisensory Information Integration in Neural Systems
Abstract
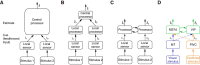
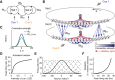
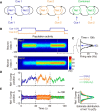
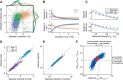
How multiple sensory cues are integrated in neural circuitry remains a challenge. The common hypothesis is that information integration might be accomplished in a dedicated multisensory integration area receiving feedforward inputs from the modalities. However, recent experimental evidence suggests that it is not a single multisensory brain area, but rather many multisensory brain areas that are simultaneously involved in the integration of information. Why many mutually connected areas should be needed for information integration is puzzling. Here, we investigated theoretically how information integration could be achieved in a distributed fashion within a network of interconnected multisensory areas. Using biologically realistic neural network models, we developed a decentralized information integration system that comprises multiple interconnected integration areas. Studying an example of combining visual and vestibular cues to infer heading direction, we show that such a decentralized system is in good agreement with anatomical evidence and experimental observations. In particular, we show that this decentralized system can integrate information optimally. The decentralized system predicts that optimally integrated information should emerge locally from the dynamics of the communication between brain areas and sheds new light on the interpretation of the connectivity between multisensory brain areas.
Significance statement: To extract information reliably from ambiguous environments, the brain integrates multiple sensory cues, which provide different aspects of information about the same entity of interest. Here, we propose a decentralized architecture for multisensory integration. In such a system, no processor is in the center of the network topology and information integration is achieved in a distributed manner through reciprocally connected local processors. Through studying the inference of heading direction with visual and vestibular cues, we show that the decentralized system can integrate information optimally, with the reciprocal connections between processers determining the extent of cue integration. Our model reproduces known multisensory integration behaviors observed in experiments and sheds new light on our understanding of how information is integrated in the brain.
Keywords: continuous attractor neural network; decentralized information integration.
Copyright © 2016 Zhang et al.
Figures
Similar articles
-
Decentralized Neural Circuits of Multisensory Information Integration in the Brain.Adv Exp Med Biol. 2024;1437:1-21. doi: 10.1007/978-981-99-7611-9_1. Adv Exp Med Biol. 2024. PMID: 38270850
-
Complementary congruent and opposite neurons achieve concurrent multisensory integration and segregation.Elife. 2019 May 23;8:e43753. doi: 10.7554/eLife.43753. Elife. 2019. PMID: 31120416 Free PMC article.
-
NeuroBayesSLAM: Neurobiologically inspired Bayesian integration of multisensory information for robot navigation.Neural Netw. 2020 Jun;126:21-35. doi: 10.1016/j.neunet.2020.02.023. Epub 2020 Mar 4. Neural Netw. 2020. PMID: 32179391
-
Neural mechanisms for synthesizing sensory information and producing adaptive behaviors.Exp Brain Res. 1998 Nov;123(1-2):124-35. doi: 10.1007/s002210050553. Exp Brain Res. 1998. PMID: 9835401 Review.
-
Optimal cue combination and landmark-stability learning in the head direction system.J Physiol. 2016 Nov 15;594(22):6527-6534. doi: 10.1113/JP272945. Epub 2016 Oct 5. J Physiol. 2016. PMID: 27479741 Free PMC article. Review.
Cited by
-
Multimodal sensory processing in Caenorhabditis elegans.Open Biol. 2018 Jun;8(6):180049. doi: 10.1098/rsob.180049. Open Biol. 2018. PMID: 29925633 Free PMC article. Review.
-
Transforming the Choice Outcome to an Action Plan in Monkey Lateral Prefrontal Cortex: A Neural Circuit Model.Neuron. 2019 Aug 7;103(3):520-532.e5. doi: 10.1016/j.neuron.2019.05.032. Epub 2019 Jun 20. Neuron. 2019. PMID: 31230761 Free PMC article.
-
Useful road maps: studying Drosophila larva's central nervous system with the help of connectomics.Curr Opin Neurobiol. 2020 Dec;65:129-137. doi: 10.1016/j.conb.2020.09.008. Epub 2020 Nov 23. Curr Opin Neurobiol. 2020. PMID: 33242722 Free PMC article. Review.
-
Development of a Bayesian Estimator for Audio-Visual Integration: A Neurocomputational Study.Front Comput Neurosci. 2017 Oct 4;11:89. doi: 10.3389/fncom.2017.00089. eCollection 2017. Front Comput Neurosci. 2017. PMID: 29046631 Free PMC article.
-
Cognitive Mapping Based on Conjunctive Representations of Space and Movement.Front Neurorobot. 2017 Nov 22;11:61. doi: 10.3389/fnbot.2017.00061. eCollection 2017. Front Neurorobot. 2017. PMID: 29213234 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
