Keratin 12 missense mutation induces the unfolded protein response and apoptosis in Meesmann epithelial corneal dystrophy
- PMID: 26758872
- PMCID: PMC4764196
- DOI: 10.1093/hmg/ddw001
Keratin 12 missense mutation induces the unfolded protein response and apoptosis in Meesmann epithelial corneal dystrophy
Abstract
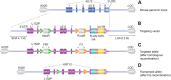
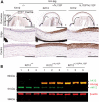
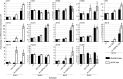
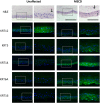
Meesmann epithelial corneal dystrophy (MECD) is a rare autosomal dominant disorder caused by dominant-negative mutations within the KRT3 or KRT12 genes, which encode the cytoskeletal protein keratins K3 and K12, respectively. To investigate the pathomechanism of this disease, we generated and phenotypically characterized a novel knock-in humanized mouse model carrying the severe, MECD-associated, K12-Leu132Pro mutation. Although no overt changes in corneal opacity were detected by slit-lamp examination, the corneas of homozygous mutant mice exhibited histological and ultrastructural epithelial cell fragility phenotypes. An altered keratin expression profile was observed in the cornea of mutant mice, confirmed by western blot, RNA-seq and quantitative real-time polymerase chain reaction. Mass spectrometry (MS) and immunohistochemistry demonstrated a similarly altered keratin profile in corneal tissue from a K12-Leu132Pro MECD patient. The K12-Leu132Pro mutation results in cytoplasmic keratin aggregates. RNA-seq analysis revealed increased chaperone gene expression, and apoptotic unfolded protein response (UPR) markers, CHOP and Caspase 12, were also increased in the MECD mice. Corneal epithelial cell apoptosis was increased 17-fold in the mutant cornea, compared with the wild-type (P < 0.001). This elevation of UPR marker expression was also observed in the human MECD cornea. This is the first reporting of a mouse model for MECD that recapitulates the human disease and is a valuable resource in understanding the pathomechanism of the disease. Although the most severe phenotype is observed in the homozygous mice, this model will still provide a test-bed for therapies not only for corneal dystrophies but also for other keratinopathies caused by similar mutations.
© The Author 2016. Published by Oxford University Press.
Figures


References
-
- Weiss J.S., Møller H.U., Aldave A.J., Seitz B., Bredrup C., Kivelä T., Munier F.L., Rapuano C.J., Nischal K.K., Kim E.K. et al. (2015) IC3D classification of corneal dystrophies—edition 2. Cornea, 34, 117–159. - PubMed
-
- Jonsson F., Byström B., Davidson A.E., Backman L.J., Kellgren T.G., Tuft S.J., Koskela T., Rydén P., Sandgren O., Danielson P. et al. (2015) Mutations in collagen, type XVII, alpha 1 (COL17A1) cause epithelial recurrent erosion dystrophy (ERED). Hum. Mutat., 36, 463–473. - PubMed
-
- Irvine A.D., Corden L.D., Swensson O., Swensson B., Moore J.E., Frazer D.G., Smith F.J., Knowlton R.G., Christophers E., Rochels R. et al. (1997) Mutations in cornea-specific keratin K3 or K12 genes cause Meesmann's corneal dystrophy. Nat. Genet., 16, 184–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

