Colonization potential to reconstitute a microbe community in patients detected early after fecal microbe transplant for recurrent C. difficile
- PMID: 26758906
- PMCID: PMC4711103
- DOI: 10.1186/s12866-015-0622-2
Colonization potential to reconstitute a microbe community in patients detected early after fecal microbe transplant for recurrent C. difficile
Abstract
Background: Fecal microbiota transplants (FMT) are an effective treatment for patients with gut microbe dysbiosis suffering from recurrent C. difficile infections. To further understand how FMT reconstitutes the patient's gut commensal microbiota, we have analyzed the colonization potential of the donor, recipient and recipient post transplant fecal samples using transplantation in gnotobiotic mice.
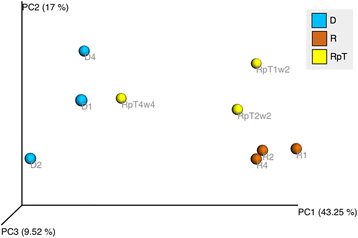
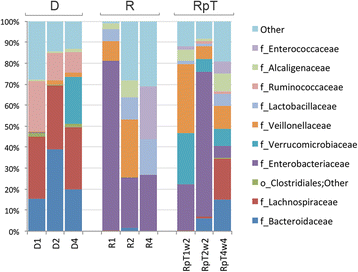
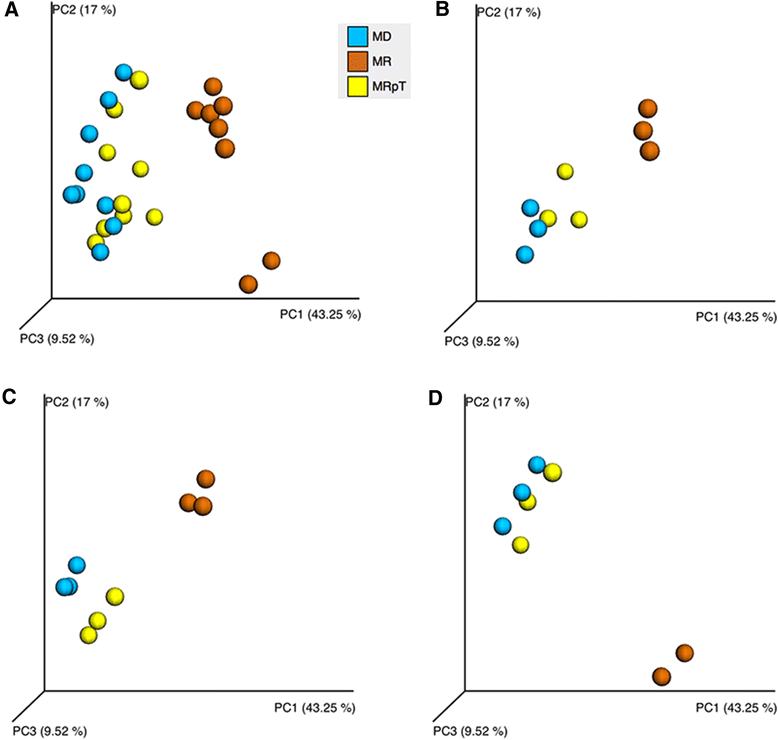
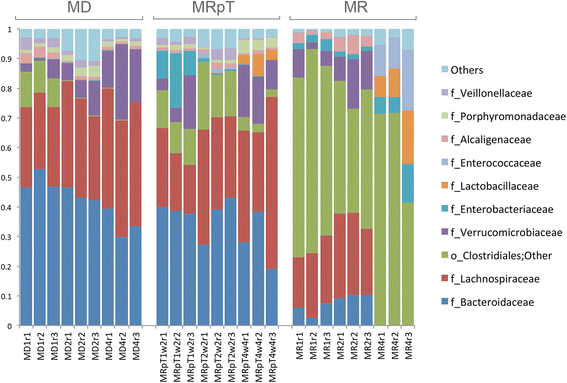
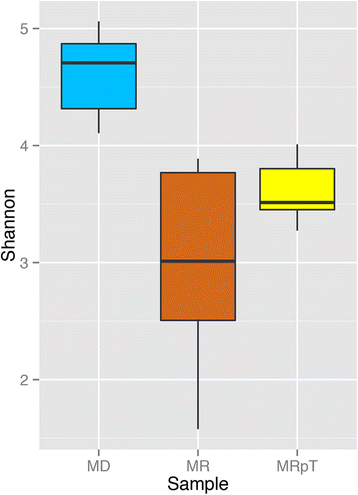
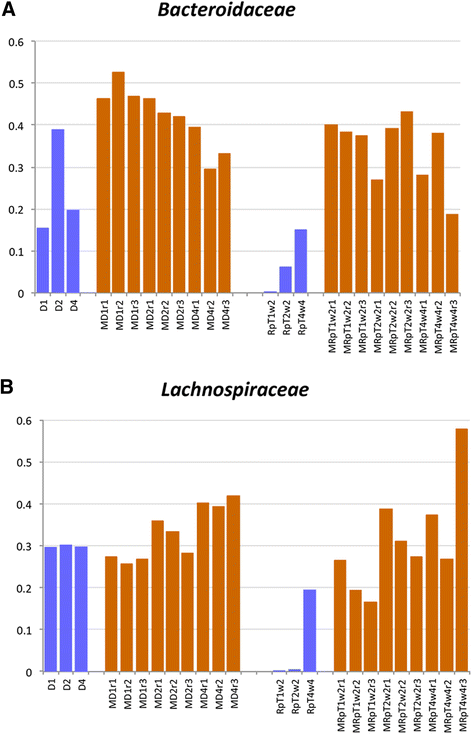
Results: A total of nine samples from three human donors, recipient's pre and post FMT were transplanted into gnotobiotic mice. Microbiome analysis of three donor fecal samples revealed the presence of a high relative abundance of commensal microbes from the family Bacteriodaceae and Lachnospiraceae that were almost absent in the three recipient pre FMT fecal samples (<0.01%). The microbe composition in gnotobiotic mice transplanted with the donor fecal samples was similar to the human samples. The recipient samples contained Enterobacteriaceae, Lactobacillaceae, Enterococcaceae in relative abundance of 43, 11, 8%, respectively. However, gnotobiotic mice transplanted with the recipient fecal samples had an average relative abundance of unclassified Clostridiales of 55%, approximately 7000 times the abundance in the recipient fecal samples prior to transplant. Microbiome analysis of fecal samples from the three patients early (2-4 weeks) after FMT revealed a microbe composition with the relative abundance of both Bacteriodaceae and Lachnospiraceae that was approximately 7% of that of the donor. In contrast, gnotobioitc mice transplanted with the fecal samples obtained from the three at early times post FMT revealed increases in the relative abundance of Bacteriodaceae and Lachnospiraceae microbe compositions to levels similar to the donor fecal samples. Furthermore, the unclassified Clostridiales in the recipient samples post FMT was reduced to an average of 10%.
Conclusion: We have used transplantation into gnotobiotic mice to evaluate the colonization potential of microbiota in FMT patients early after transplant. The commensal microbes present at early times post FMT out competed non-commensal microbes (e.g. such as unclassified Clostridiales) for niche space. The selective advantage of these commensal microbes to occupy niches in the gastrointestinal tract helps to explain the success of FMT to reconstitute the gut microbe community of patients with recurrent C. difficile infections.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

