Genetic treatment of a molecular disorder: gene therapy approaches to sickle cell disease
- PMID: 26758916
- PMCID: PMC4760089
- DOI: 10.1182/blood-2015-09-618587
Genetic treatment of a molecular disorder: gene therapy approaches to sickle cell disease
Abstract
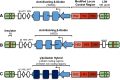
Effective medical management for sickle cell disease (SCD) remains elusive. As a prevalent and severe monogenic disorder, SCD has been long considered a logical candidate for gene therapy. Significant progress has been made in moving toward this goal. These efforts have provided substantial insight into the natural regulation of the globin genes and illuminated challenges for genetic manipulation of the hematopoietic system. The initial γ-retroviral vectors, next-generation lentiviral vectors, and novel genome engineering and gene regulation approaches each share the goal of preventing erythrocyte sickling. After years of preclinical studies, several clinical trials for SCD gene therapies are now open. This review focuses on progress made toward achieving gene therapy, the current state of the field, consideration of factors that may determine clinical success, and prospects for future development.
© 2016 by The American Society of Hematology.
Figures

References
-
- Stamatoyannopoulos G. The molecular basis of hemoglobin disease. Annu Rev Genet. 1972;6:47–70. - PubMed
-
- Platt OS, Rosenstock W, Espeland MA. Influence of sickle hemoglobinopathies on growth and development. N Engl J Med. 1984;311(1):7–12. - PubMed
-
- Madigan C, Malik P. Pathophysiology and therapy for haemoglobinopathies. Part I: sickle cell disease. Expert Rev Mol Med. 2006;8(9):1–23. - PubMed
-
- Creary M, Williamson D, Kulkarni R. Sickle cell disease: current activities, public health implications, and future directions. J Womens Health (Larchmt) 2007;16(5):575–582. - PubMed
-
- Locatelli F, Kabbara N, Ruggeri A, et al. Eurocord and European Blood and Marrow Transplantation (EBMT) group. Outcome of patients with hemoglobinopathies given either cord blood or bone marrow transplantation from an HLA-identical sibling. Blood. 2013;122(6):1072–1078. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

