Beyond hydroxyurea: new and old drugs in the pipeline for sickle cell disease
- PMID: 26758919
- PMCID: PMC4760087
- DOI: 10.1182/blood-2015-09-618553
Beyond hydroxyurea: new and old drugs in the pipeline for sickle cell disease
Abstract
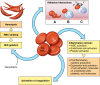
Despite Food and Drug Administration (FDA) approval of hydroxyurea to reduce the frequency of vaso-occlusive episodes, sickle cell disease (SCD) has continued to be treated primarily with analgesics for pain relief. However, elucidation of the multiple pathophysiologic mechanisms leading to vaso-occlusion and tissue injury in SCD has now resulted in a burgeoning effort to identify new treatment modalities to prevent or ameliorate the consequences of the disease. Development of new drugs as well as investigation of drugs previously used in other settings have targeted cell adhesion, inflammatory pathways, upregulation of hemoglobin F, hemoglobin polymerization and sickling, coagulation, and platelet activation. Although these efforts have not yet yielded drugs ready for FDA approval, several early studies have been extremely encouraging. Moreover, the marked increase in clinical pharmaceutical research addressing SCD and the new and old drugs in the pipeline make it reasonable to expect that we will soon have new treatments for SCD.
© 2016 by The American Society of Hematology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

