Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss
- PMID: 26759138
- PMCID: PMC4755442
- DOI: 10.1093/humrep/dev329
Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss
Abstract
Study question: What is the association between daily preconception-initiated low-dose aspirin (LDA) treatment and very early pregnancy losses or euploid (chromosomally normal) losses among women with one to two prior losses?
Summary answer: Daily LDA initiated preconception was not associated with the rate or type of pregnancy loss among women with a history of one to two prior pregnancy losses.
What is known already: LDA is often used to treat recurrent pregnancy loss with reductions in pregnancy loss generally only observed among women with antiphospholipid antibodies, and null associations observed among women without antiphospholipid antibodies. We previously evaluated the association between LDA and pregnancy loss overall among women with one to two prior losses in the Effects of Aspirin in Gestation and Reproduction (EAGeR) trial and found no association, though did not distinguish between potential effects at different stages of pregnancy loss, including implantation failure, or between euploid and aneuploid losses.
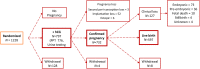
Study design, size, duration: The EAGeR trial was a multi-site prospective block-randomized double-blind placebo-controlled trial. In total, 1228 women were randomized to daily LDA (81 mg/day) plus folic acid (400 mcg/day), or placebo plus folic acid. Participants were assigned study drug for less than or equal to six menstrual cycles or if they conceived, throughout pregnancy with study drug discontinued at 36 weeks gestation. This analysis includes additional outcome information obtained from chart abstractions after the completion of the trial, as well as testing of stored urine for measurement of hCG and detection of very early pregnancy losses, and karyotyping of the products of conception for assessment of aneuploidy of the losses.
Participants, setting, methods: Women aged 18-40 with a history of one to two prior losses and actively trying to conceive were randomized (n = 615 LDA and n = 613 placebo) at four clinical centers in the USA (2007-2011). Log-binomial regression was used to estimate risk ratios under the intent-to-treat approach.
Main results and the role of chance: Daily LDA initiated preconception was not associated with clinically recognized pregnancy losses or implantation failures among women with proved fecundity and a history of one to two prior losses. Specifically, 1088 (88.6%) women completed the trial with 797 having an hCG detected pregnancy (64.9%). Overall there were 133 clinical losses (12.7% LDA versus 11.8% placebo, P = 0.71) and 55 implantation failures (5.2% LDA versus 4.9% placebo, P = 0.89). No differences were found in rate of euploid losses (RR 1.11, 95% confidence interval: 0.99, 1.26).
Limitations, reasons for caution: Generalizability of these findings is limited to women with a history of one to two prior losses, and may further be limited to women of white race with higher socioeconomic status as given the rigors of the study protocol participants tended to be white and have higher incomes and more education. We were also missing karyotype information on approximately one-third of the clinically recognized pregnancy losses, which may limit our power to detect effects on euploid losses, though detailed sensitivity analysis showed similar results.
Wider implications of the findings: Our data do not support the general use of LDA to decrease pregnancy loss and further demonstrate no increased risk of loss for women on LDA treatment.
Study funding/competing interests: This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland (Contract Nos. HHSN267200603423, HHSN267200603424, HHSN267200603426). The authors have no conflicts of interest.
Trial registration number: The trial was registered at ClinicalTrials.gov #NCT00467363.
Trial registration date: 27 April 2007.
Date of first patient's enrollment: 15 June 2007.
Keywords: conception; fertility; live birth; low-dose aspirin; pregnancy loss.
Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology 2016. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures

References
-
- Dentali F, Ageno W, Rezoagli E, Rancan E, Squizzato A, Middeldorp S, Margaglione M, Grandone E. Low-dose aspirin for in vitro fertilization or intracytoplasmic sperm injection: a systematic review and a meta-analysis of the literature. J Thromb Haemost 2012;10:2075–2085. - PubMed
-
- Di Nisio M, Peters L, Middeldorp S. Anticoagulants for the treatment of recurrent pregnancy loss in women without antiphospholipid syndrome. Cochrane Database Syst Rev 2005;2:CD004734. - PubMed
-
- Empson M, Lassere M, Craig JC, Scott JR. Recurrent pregnancy loss with antiphospholipid antibody: a systematic review of therapeutic trials. Obstet Gynecol 2002;99:135–144. - PubMed
-
- Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol 2002;100:408–413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

