Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress
- PMID: 26759232
- PMCID: PMC4794393
- DOI: 10.1158/0008-5472.CAN-15-1591
Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress
Abstract
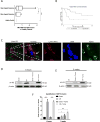
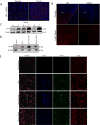
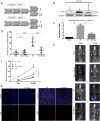
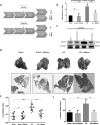
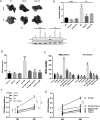
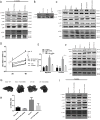
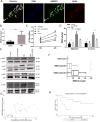
Risks of tumor recurrence after surgical resection have been known for decades, but the mechanisms underlying treatment failures remain poorly understood. Neutrophils, first-line responders after surgical stress, may play an important role in linking inflammation to cancer progression. In response to stress, neutrophils can expel their protein-studded chromatin to form local snares known as neutrophil extracellular traps (NET). In this study, we asked whether, as a result of its ability to ensnare moving cells, NET formation might promote metastasis after surgical stress. Consistent with this hypothesis, in a cohort of patients undergoing attempted curative liver resection for metastatic colorectal cancer, we observed that increased postoperative NET formation was associated with a >4-fold reduction in disease-free survival. In like manner, in a murine model of surgical stress employing liver ischemia-reperfusion, we observed an increase in NET formation that correlated with an accelerated development and progression of metastatic disease. These effects were abrogated by inhibiting NET formation in mice through either local treatment with DNAse or inhibition of the enzyme peptidylarginine deaminase, which is essential for NET formation. In growing metastatic tumors, we found that intratumoral hypoxia accentuated NET formation. Mechanistic investigations in vitro indicated that mouse neutrophil-derived NET triggered HMGB1 release and activated TLR9-dependent pathways in cancer cells to promote their adhesion, proliferation, migration, and invasion. Taken together, our findings implicate NET in the development of liver metastases after surgical stress, suggesting that their elimination may reduce risks of tumor relapse.
©2016 American Association for Cancer Research.
Conflict of interest statement
Figures
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015 Jan-Feb;65(1):5–29. - PubMed
-
- Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Aug 1;27(22):3677–83. - PMC - PubMed
-
- Murthy SM, Goldschmidt RA, Rao LN, Ammirati M, Buchmann T, Scanlon EF. The influence of surgical trauma on experimental metastasis. Cancer. 1989 Nov 15;64(10):2035–44. - PubMed
-
- van der Bij GJ, Oosterling SJ, Beelen RH, Meijer S, Coffey JC, van Egmond M. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Annals of surgery. 2009 May;249(5):727–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

