Intracortical Microstimulation Maps of Motor, Somatosensory, and Posterior Parietal Cortex in Tree Shrews (Tupaia belangeri) Reveal Complex Movement Representations
- PMID: 26759478
- PMCID: PMC6075024
- DOI: 10.1093/cercor/bhv329
Intracortical Microstimulation Maps of Motor, Somatosensory, and Posterior Parietal Cortex in Tree Shrews (Tupaia belangeri) Reveal Complex Movement Representations
Abstract
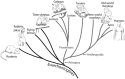
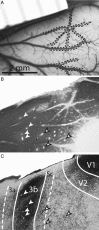
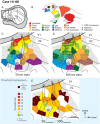
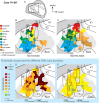
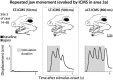
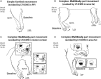
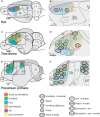
Long-train intracortical microstimulation (LT-ICMS) is a popular method for studying the organization of motor and posterior parietal cortex (PPC) in mammals. In primates, LT-ICMS evokes both multijoint and multiple-body-part movements in primary motor, premotor, and PPC. In rodents, LT-ICMS evokes complex movements of a single limb in motor cortex. Unfortunately, very little is known about motor/PPC organization in other mammals. Tree shrews are closely related to both primates and rodents and could provide insights into the evolution of complex movement domains in primates. The present study investigated the extent of cortex in which movements could be evoked with ICMS and the characteristics of movements elicited using both short train (ST) and LT-ICMS in tree shrews. We demonstrate that LT-ICMS and ST-ICMS maps are similar, with the movements elicited with ST-ICMS being truncated versions of those elicited with LT-ICMS. In addition, LT-ICMS-evoked complex movements within motor cortex similar to those in rodents. More complex movements involving multiple body parts such as the hand and mouth were also elicited in motor cortex and PPC, as in primates. Our results suggest that complex movement networks present in PPC and motor cortex were present in mammals prior to the emergence of primates.
Keywords: evolution; grasping; motor cortex; primate; reaching.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures





References
-
- Asanuma H , Sakata H . 1967. . Functional organization of the cortical efferent system examined with focal depth stimulation in cats . J Neurophysiol . 30 : 35 – 54 .
-
- Brecht M , Krauss A , Muhammad S , Sinai-Esfahani L , Bellanca S , Margie TW . 2004. . Organization of rat vibrissa motor cortex and adjacent areas according to cytoarchitectonics, microstimulation, and intracellular stimulation of identified cells . J Comp Neurol . 479 : 360 – 373 . - PubMed
-
- Burish MJ , Stepniewska I , Kaas JH . 2008. . Microstimulation and architectonics of frontoparietal cortex in common marmosets ( Callithrix jacchus ) . J Comp Neurol . 507 : 1151 – 1168 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

