Interaction between neonatal maternal deprivation and serum leptin levels on metabolism, pubertal development, and sexual behavior in male and female rats
- PMID: 26759712
- PMCID: PMC4710050
- DOI: 10.1186/s13293-015-0054-6
Interaction between neonatal maternal deprivation and serum leptin levels on metabolism, pubertal development, and sexual behavior in male and female rats
Abstract
Background: Maternal deprivation (MD) during neonatal life can have long-term effects on metabolism and behavior, with males and females responding differently. We previously reported that MD during 24 h at postnatal day (PND) 9 blocks the physiological neonatal leptin surge in both sexes. It is known that modifications in neonatal leptin levels can affect metabolism in adulthood. Thus, we hypothesized that at least some of the long-term metabolic changes that occur in response to MD are due to the decline in serum leptin during this critical period of development. Hence, we predicted that treatment with leptin during MD would normalize these metabolic changes, with this response also differing between the sexes.
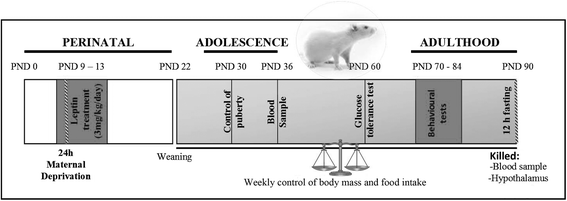
Methods: MD was carried-out in Wistar rats for 24 h on PND9. Control and MD rats of both sexes were treated from PND 9 to 13 with leptin (3 mg/kg/day sc) or vehicle. Weight gain, food intake, glucose tolerance, and pubertal onset were monitored. Sexual behavior was analyzed in males. Rats were killed at PND90, and serum hormones and hypothalamic neuropeptides involved in metabolic control and reproduction were measured. Results were analyzed by three-way analysis of covariance using sex, MD, and leptin treatment as factors and litter as the covariate and employing repeated measures where appropriate.
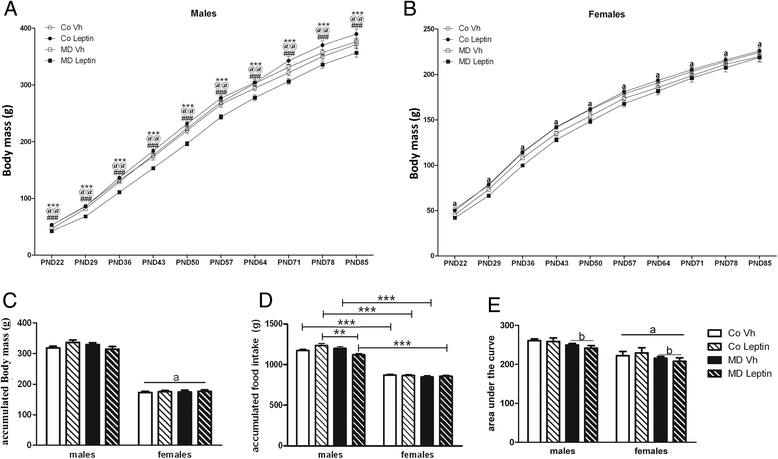
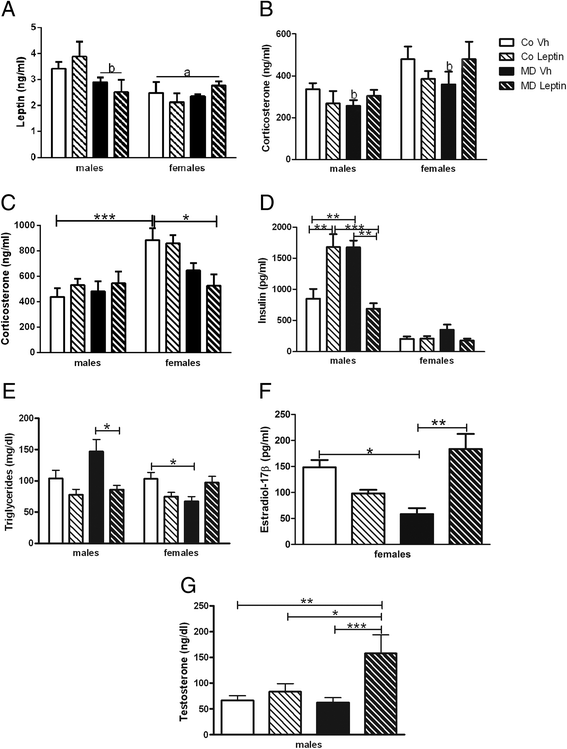
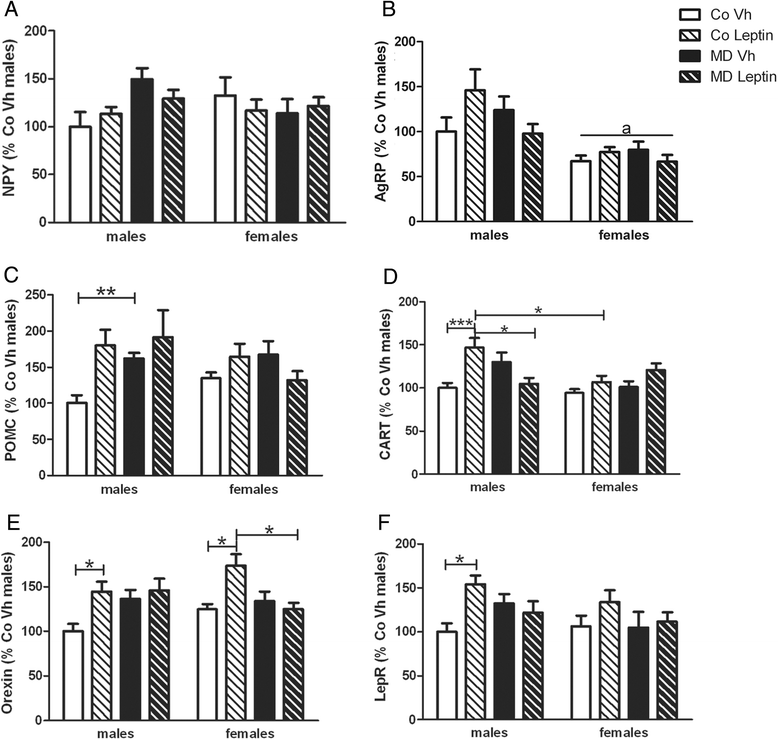
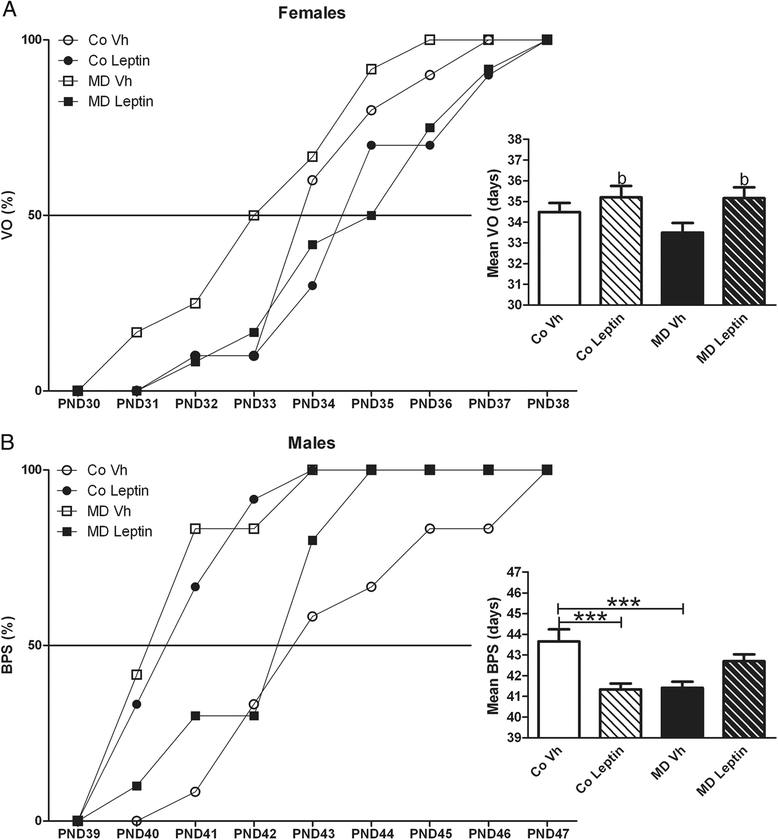
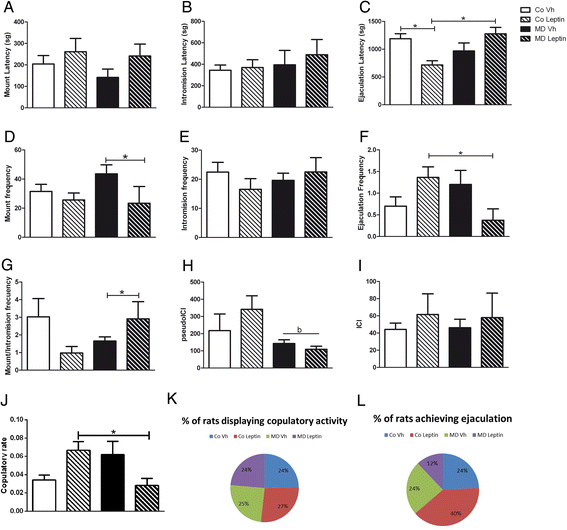
Results: In males, MD advanced the external signs of puberty and increased serum insulin and triglyceride levels and hypothalamic proopiomelanocortin mRNA levels at PND90. Neonatal leptin treatment normalized these effects. In contrast, MD decreased circulating triglycerides, as well as estradiol levels, in females at PND90 and these changes were also normalized by neonatal leptin treatment. Neonatal leptin treatment also had long-term effects in control rats as it advanced the external signs of puberty in control males, but delayed them in females. Neonatal leptin treatment increased serum insulin and hypothalamic mRNA levels of the leptin receptor and cocaine- and amphetamine-regulated transcript in control males and increased orexin mRNA levels in controls of both sexes. Although pubertal onset in males was advanced by either MD or neonatal leptin treatment in males and delayed by leptin treatment in females, the mRNA levels of hypothalamic neuropeptides and receptors related to reproduction were not affected by MD or neonatal leptin treatment in either sex at PND90.
Conclusions: These findings indicate that some of the long-term changes in metabolic and reproductive parameters induced by MD, such as advanced pubertal onset and increased hypothalamic proopiomelanocortin (POMC) expression, hyperinsulinemia, and hypertriglyceridemia in adult males and decreased serum triglyceride and estradiol levels in females, are most likely due to the decrease in leptin levels during the period of MD.
Keywords: Hypothalamus; Neonatal leptin surge; Neuropeptides; Puberty; Reproduction; Sexual dimorphism; Weight gain.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

