Low expression of estrogen receptor β in T lymphocytes and high serum levels of anti-estrogen receptor α antibodies impact disease activity in female patients with systemic lupus erythematosus
- PMID: 26759713
- PMCID: PMC4709986
- DOI: 10.1186/s13293-016-0057-y
Low expression of estrogen receptor β in T lymphocytes and high serum levels of anti-estrogen receptor α antibodies impact disease activity in female patients with systemic lupus erythematosus
Abstract
Background: Current evidence indicates that estrogens, in particular 17β-estradiol (E2), play a crucial role in the gender bias of autoimmune diseases although the underlying molecular mechanisms have not yet been fully elucidated. Immune cells have estrogen receptors (ERs), i.e., ERα and ERβ, that play pro- and anti-inflammatory functions, respectively, and the presence of one estrogen receptor (ER) subtype over the other might change estrogen effects, promoting or dampening inflammation. In this study, we contributed to define the influences of E2 on T cells from female patients with systemic lupus erythematosus (SLE), a representative autoimmune disease characterized by a higher prevalence in women than in men (female/male ratio 9:1). Particularly, our aim was to evaluate whether alterations of ERα and ERβ expression in T cells from female SLE patients may impact lymphocyte sensitivity to E2 and anti-ERα antibody (anti-ERα Ab) stimulation interfering with cell signaling and display a direct clinical effect.
Methods: Sixty-one premenopausal female patients with SLE and 40 age-matched healthy donors were recruited. Patients were divided into two groups based on the SLE Disease Activity Index 2000 (SLEDAI-2K) (i.e., <6 and ≥6). ER expression was evaluated in T lymphocytes by flow cytometry, immunofluorescence, and Western blot analyses. Serum anti-ERα Ab levels were analyzed by enzyme-linked immunosorbent assay (ELISA). ER-dependent signaling pathways were measured by a phosphoprotein detection kit.
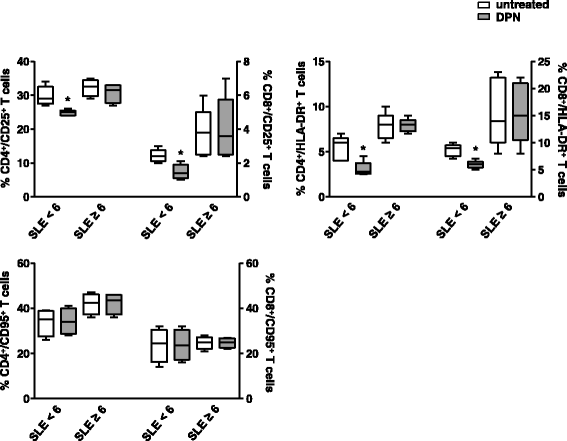
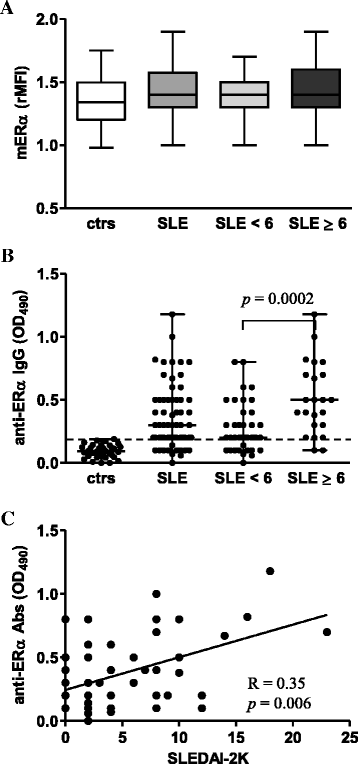
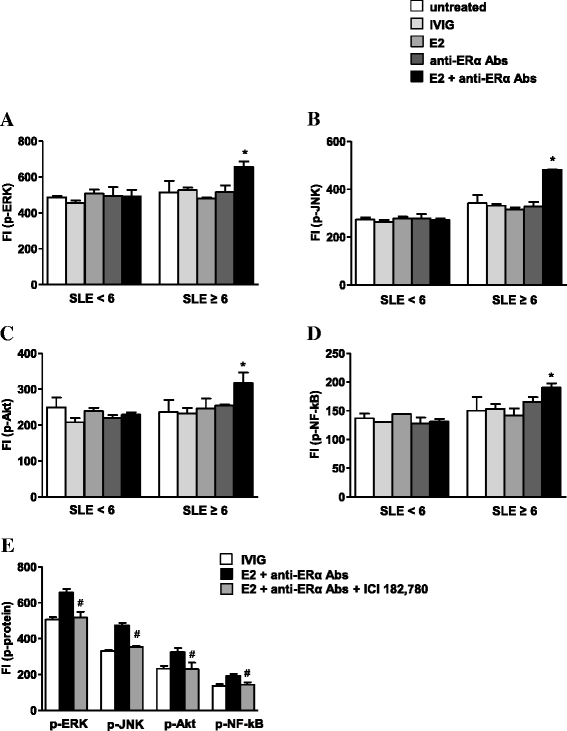
Results: Intracellular ERβ expression was significantly lower in T cells from patients with SLEDAI-2K ≥6 as compared with healthy donors and patients with SLEDAI-2K <6 and negatively correlated with disease activity. The expression of intracellular and membrane-associated-ERα was similar in SLE and control T cells. ER-dependent signaling pathways were activated in T cells from SLE patients with SLEDAI-2K ≥6, but not with SLEDAI-2K <6, when both membrane and intracellular ERs were stimulated by co-treatment with E2 and anti-ERα Abs.
Conclusions: Our results demonstrate an altered ER profile in SLE patients, possibly contributing to SLE pathogenesis and interfering with clinical activity, and highlight the potential exploitation of T cell-associated ERβ as a biomarker of disease activity.
Keywords: Anti-ERα antibodies; Estrogen; Estrogen receptor; Gender; Immunity; Systemic lupus erythematosus; T lymphocytes.
Figures

Similar articles
-
Autoantibodies to estrogen receptor α interfere with T lymphocyte homeostasis and are associated with disease activity in systemic lupus erythematosus.Arthritis Rheum. 2012 Mar;64(3):778-87. doi: 10.1002/art.33400. Arthritis Rheum. 2012. PMID: 21968947
-
Gender Bias in Human Systemic Lupus Erythematosus: A Problem of Steroid Receptor Action?Front Immunol. 2018 Mar 28;9:611. doi: 10.3389/fimmu.2018.00611. eCollection 2018. Front Immunol. 2018. PMID: 29643853 Free PMC article. Clinical Trial.
-
Expression of human T cell immunoglobulin domain and mucin-3 (TIM-3) and TIM-3 ligands in peripheral blood from patients with systemic lupus erythematosus.Arch Dermatol Res. 2016 Oct;308(8):553-61. doi: 10.1007/s00403-016-1665-4. Epub 2016 Jul 9. Arch Dermatol Res. 2016. PMID: 27394439
-
Estrogen receptors in immunity and autoimmunity.Clin Rev Allergy Immunol. 2011 Feb;40(1):66-73. doi: 10.1007/s12016-010-8203-5. Clin Rev Allergy Immunol. 2011. PMID: 20352526 Review.
-
Gender differences in autoimmunity: molecular basis for estrogen effects in systemic lupus erythematosus.Int Immunopharmacol. 2001 Jun;1(6):1009-24. doi: 10.1016/s1567-5769(01)00046-7. Int Immunopharmacol. 2001. PMID: 11407298 Review.
Cited by
-
Serum miR-342-3p Acts as a Biomarker for Systemic Lupus Erythematosus and Participates in the Disease Progression.Clin Cosmet Investig Dermatol. 2023 Jan 5;16:39-46. doi: 10.2147/CCID.S378985. eCollection 2023. Clin Cosmet Investig Dermatol. 2023. PMID: 36636634 Free PMC article.
-
Sex bias in lymphocytes: Implications for autoimmune diseases.Front Immunol. 2022 Nov 24;13:945762. doi: 10.3389/fimmu.2022.945762. eCollection 2022. Front Immunol. 2022. PMID: 36505451 Free PMC article. Review.
-
What we do and do not know about women and kidney diseases; questions unanswered and answers unquestioned: reflection on World Kidney Day and International Women's Day.J Nephrol. 2018 Apr;31(2):173-184. doi: 10.1007/s40620-018-0474-6. Epub 2018 Feb 20. J Nephrol. 2018. PMID: 29464527
-
Sex Steroids Effects on Asthma: A Network Perspective of Immune and Airway Cells.Cells. 2022 Jul 19;11(14):2238. doi: 10.3390/cells11142238. Cells. 2022. PMID: 35883681 Free PMC article. Review.
-
GPER1-mediated suppression of acute cellular rejection in murine cervical heart transplantation by estradiol.J Cardiothorac Surg. 2025 Apr 17;20(1):208. doi: 10.1186/s13019-025-03432-8. J Cardiothorac Surg. 2025. PMID: 40247327 Free PMC article.
References
-
- Pierdominici M, Ortona E. Estrogen impact on autoimmunity onset and progression: the paradigm of systemic lupus erythematosus. International Trends in Immunity. 2013;1:24–34.
LinkOut - more resources
Full Text Sources
Other Literature Sources

