Spatial sexual dimorphism of X and Y homolog gene expression in the human central nervous system during early male development
- PMID: 26759715
- PMCID: PMC4710049
- DOI: 10.1186/s13293-015-0056-4
Spatial sexual dimorphism of X and Y homolog gene expression in the human central nervous system during early male development
Abstract
Background: Renewed attention has been directed to the functions of the Y chromosome in the central nervous system during early human male development, due to the recent proposed involvement in neurodevelopmental diseases. PCDH11Y and NLGN4Y are of special interest because they belong to gene families involved in cell fate determination and formation of dendrites and axon.
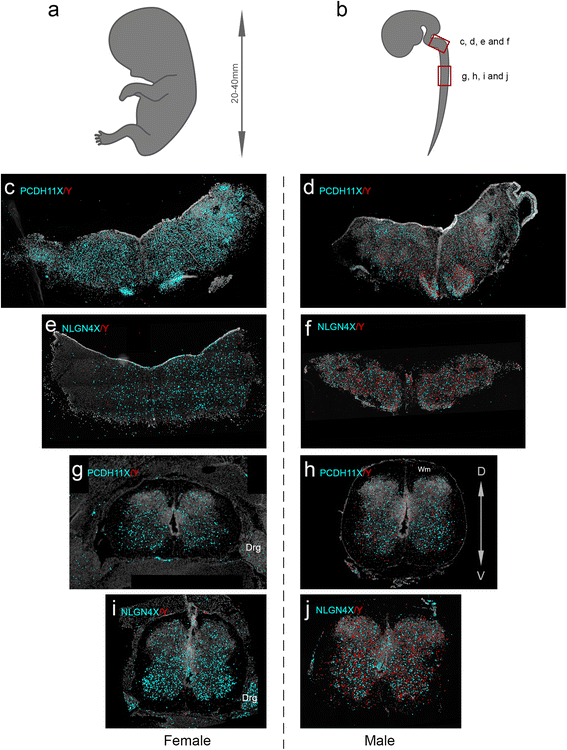
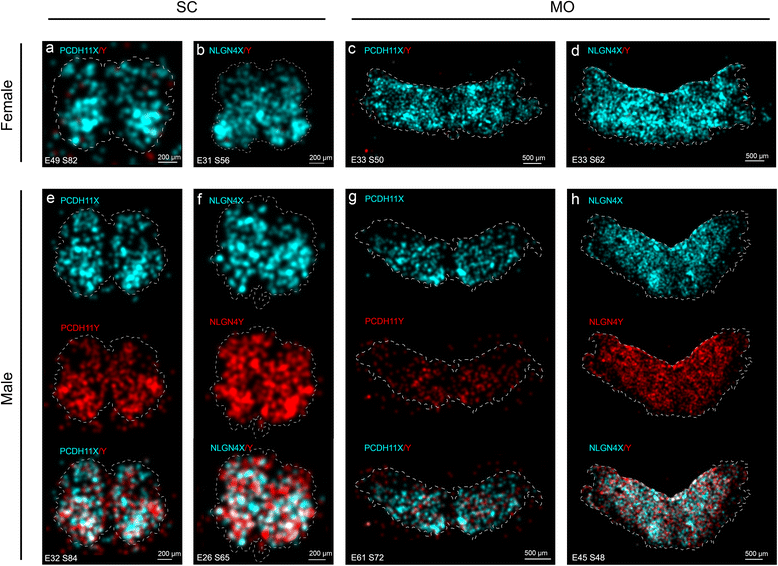
Methods: We used RNA sequencing, immunocytochemistry and a padlock probing and rolling circle amplification strategy, to distinguish the expression of X and Y homologs in situ in the human brain for the first time. To minimize influence of androgens on the sex differences in the brain, we focused our investigation to human embryos at 8-11 weeks post-gestation.
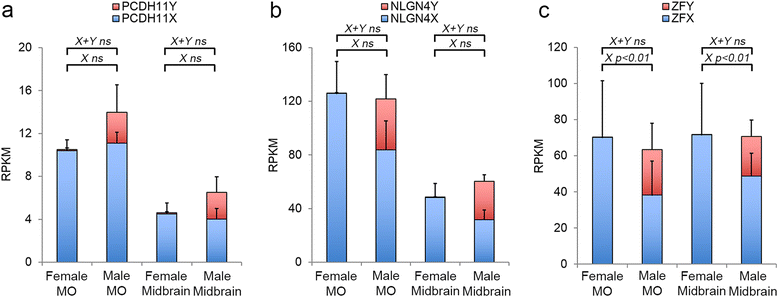
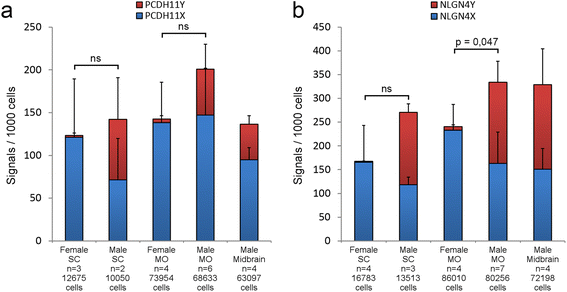
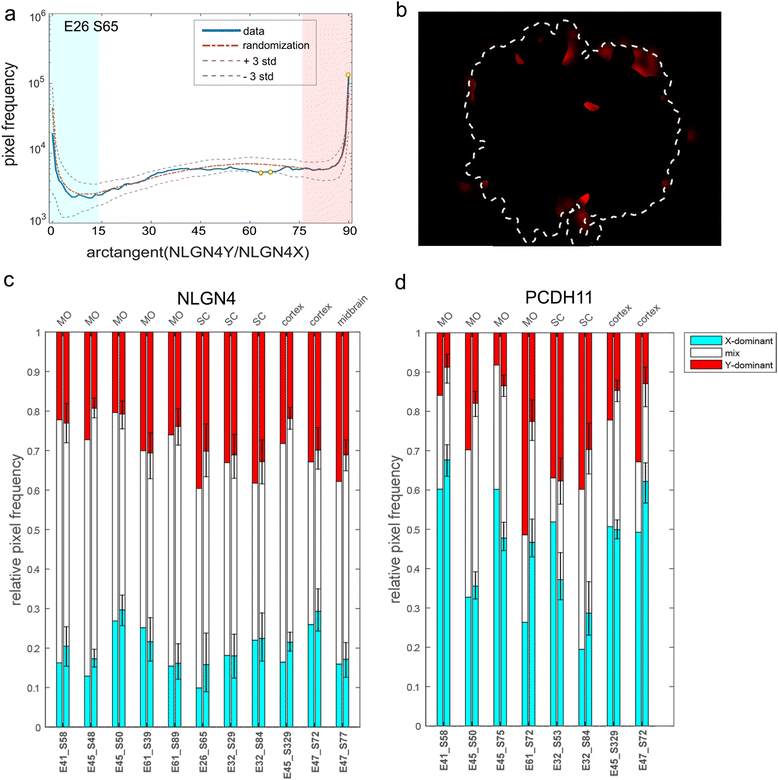
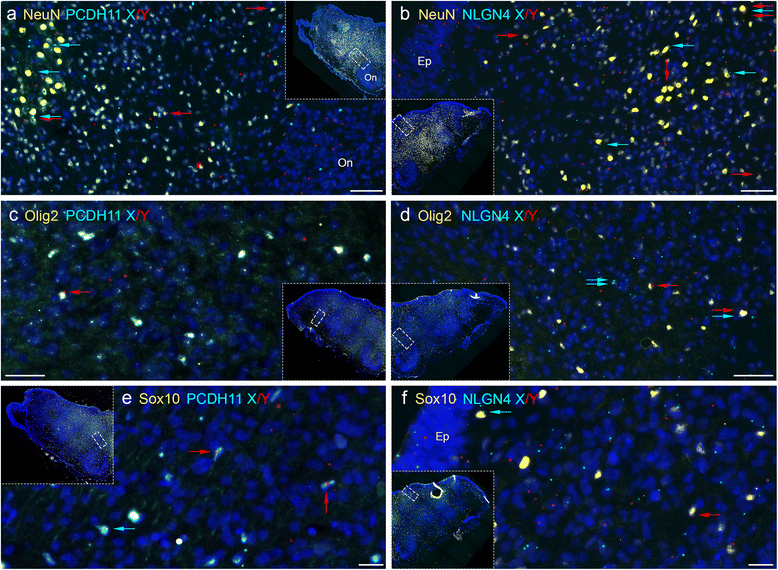
Results: We found that the X- and Y-encoded genes are expressed in specific and heterogeneous cellular sub-populations of both glial and neuronal origins. More importantly, we found differential distribution patterns of X and Y homologs in the male developing central nervous system.
Conclusions: This study has visualized the spatial distribution of PCDH11X/Y and NLGN4X/Y in human developing nervous tissue. The observed spatial distribution patterns suggest the existence of an additional layer of complexity in the development of the male CNS.
Keywords: Brain; Cortex; Female; Gene expression; Human embryo development; ISLET1; Male; Medulla oblongata; NLGN4X; NLGN4Y; NeuN; Neuroligin; OLIG2; PCDH11X; PCDH11Y; Protocadherin; Rolling circle amplification; SOX10; Sex differences; Spinal cord; X chromosome; Y chromosome.
Figures

Similar articles
-
A paradigmatic autistic phenotype associated with loss of PCDH11Y and NLGN4Y genes.BMC Med Genomics. 2021 Apr 8;14(1):98. doi: 10.1186/s12920-021-00934-x. BMC Med Genomics. 2021. PMID: 33832486 Free PMC article.
-
Inactivation status of PCDH11X: sexual dimorphisms in gene expression levels in brain.Hum Genet. 2006 Apr;119(3):267-75. doi: 10.1007/s00439-006-0134-0. Epub 2006 Jan 20. Hum Genet. 2006. PMID: 16425037
-
Protocadherin 11X/Y a human-specific gene pair: an immunohistochemical survey of fetal and adult brains.Cereb Cortex. 2013 Aug;23(8):1933-41. doi: 10.1093/cercor/bhs181. Epub 2012 Jun 28. Cereb Cortex. 2013. PMID: 22744706 Free PMC article.
-
Transcriptional sexual dimorphism during preimplantation embryo development and its consequences for developmental competence and adult health and disease.Reproduction. 2011 May;141(5):563-70. doi: 10.1530/REP-10-0482. Epub 2011 Feb 21. Reproduction. 2011. PMID: 21339284 Review.
-
Establishing sexual dimorphism in humans.Coll Antropol. 2006 Sep;30(3):653-8. Coll Antropol. 2006. PMID: 17058539 Review.
Cited by
-
Stage 1 Registered Report: Variation in neurodevelopmental outcomes in children with sex chromosome trisomies: protocol for a test of the double hit hypothesis.Wellcome Open Res. 2018 Feb 12;3:10. doi: 10.12688/wellcomeopenres.13828.2. eCollection 2018. Wellcome Open Res. 2018. PMID: 29744390 Free PMC article.
-
Temporal sex specific brain gene expression pattern during early rat embryonic development.Front Cell Dev Biol. 2024 Jun 19;12:1343800. doi: 10.3389/fcell.2024.1343800. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38961864 Free PMC article.
-
Genetics of the human Y chromosome and its association with male infertility.Reprod Biol Endocrinol. 2018 Feb 17;16(1):14. doi: 10.1186/s12958-018-0330-5. Reprod Biol Endocrinol. 2018. PMID: 29454353 Free PMC article. Review.
-
Genes that escape from X-chromosome inactivation: Potential contributors to Klinefelter syndrome.Am J Med Genet C Semin Med Genet. 2020 Jun;184(2):226-238. doi: 10.1002/ajmg.c.31800. Epub 2020 May 22. Am J Med Genet C Semin Med Genet. 2020. PMID: 32441398 Free PMC article. Review.
-
Transcriptomic sex differences in early human fetal brain development.Commun Biol. 2025 Apr 25;8(1):664. doi: 10.1038/s42003-025-08070-3. Commun Biol. 2025. PMID: 40281071 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

