Cellular and subcellular oxidative stress parameters following severe spinal cord injury
- PMID: 26760911
- PMCID: PMC4712315
- DOI: 10.1016/j.redox.2015.12.011
Cellular and subcellular oxidative stress parameters following severe spinal cord injury
Abstract
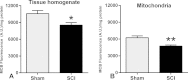
The present study undertook a comprehensive assessment of the acute biochemical oxidative stress parameters in both cellular and, notably, mitochondrial isolates following severe upper lumbar contusion spinal cord injury (SCI) in adult female Sprague Dawley rats. At 24h post-injury, spinal cord tissue homogenate and mitochondrial fractions were isolated concurrently and assessed for glutathione (GSH) content and production of nitric oxide (NO(•)), in addition to the presence of oxidative stress markers 3-nitrotyrosine (3-NT), protein carbonyl (PC), 4-hydroxynonenal (4-HNE) and lipid peroxidation (LPO). Moreover, we assessed production of superoxide (O2(•-)) and hydrogen peroxide (H2O2) in mitochondrial fractions. Quantitative biochemical analyses showed that compared to sham, SCI significantly lowered GSH content accompanied by increased NO(•) production in both cellular and mitochondrial fractions. SCI also resulted in increased O2(•-) and H2O2 levels in mitochondrial fractions. Western blot analysis further showed that reactive oxygen/nitrogen species (ROS/RNS) mediated PC and 3-NT production were significantly higher in both fractions after SCI. Conversely, neither 4-HNE levels nor LPO formation were increased at 24h after injury in either tissue homogenate or mitochondrial fractions. These results indicate that by 24h post-injury ROS-induced protein oxidation is more prominent compared to lipid oxidation, indicating a critical temporal distinction in secondary pathophysiology that is critical in designing therapeutic approaches to mitigate consequences of oxidative stress.
Keywords: 3-Nitrotyrosine; 4-Hydroxynonenal; Mitochondria; Protein carbonyl; RNS; ROS.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures







References
-
- Fatima G., Sharma V.P., Das S.K., Mahdi A.A. Oxidative stress and antioxidative parameters in patients with spinal cord injury: implications in the pathogenesis of disease. Spinal Cord. 2015;53:3–6. - PubMed
-
- Rabchevsky A.G., Patel S.P., Springer J.E. Pharmacological interventions for spinal cord injury: where do we stand? How might we step forward? Pharmacol. Ther. 2011;132:15–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

