Rapid Determination of L-carnitine in Infant and Toddler Formulas by Liquid Chromatography Tandem Mass Spectrometry
- PMID: 26761670
- PMCID: PMC4662188
- DOI: 10.5851/kosfa.2014.34.6.749
Rapid Determination of L-carnitine in Infant and Toddler Formulas by Liquid Chromatography Tandem Mass Spectrometry
Abstract
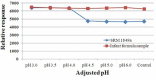
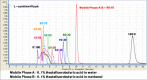
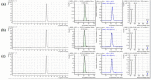
A rapid and simple analytical method for L-carnitine was developed for infant and toddler formulas by liquid chromatography tandem mass spectrometry (LC-MS/MS). A 0.3 g of infant formula and toddler formula sample was mixed in a 50 mL conical tube with 9 mL water and 1 mL 0.1 M hydrochloric acid (HCl) to chemical extraction. Then, chloroform was used for removing a lipid fraction. After centrifuged, L-carnitine was separated and quantified using LC-MS/MS with electrospray ionization (ESI) mode. The precursor ion for L-carnitine was m/z 162, and product ions were m/z 103 (quantitative) and m/z 85 (qualitative), respectively. The results for spiked recovery test were in the range of 93.18-95.64% and the result for certified reference material (SRM 1849a) was within the range of the certificated values. This method could be implemented in many laboratories that require time and labor saving.
Keywords: L-carnitine; LC-MS/MS; analytical method; infant formula; toddler formula.
Figures

References
-
- Andrieux P., Kilinc T., Perrin C., Campos-Giménez E. Simultaneous determination of free carnitine and total choline by liquid chromatography/mass spectrometry in infant formula and health-care products: single-laboratory validation. J. AOAC Int. (2008);91:777–785. - PubMed
-
- AOAC International. AOAC Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals. Association of Official Analytical Chemists; Gaithersburg, MD, USA: (2002).
-
- AOAC Official Method 999.14. Choline in Infant Formula and Milk to the Determination of Choline in Dietary Supplements. Enzymatic colorimetric method, Final Action 2003. (2003). - PubMed
-
- Codex guideline. Draft Guidelines for the Design and Implementation of National Regulatory Food Safety Assurance Programs Associated with the Use of Veterinary Drugs in Food Producing Animals. Codex Alimentarius Commission; Rome, Italy: (2007).
-
- Official Journal of the European Communities. Lisbon, Portugal: (2002). Commission decision of 12 August 2002, Implementing council directive 96/23/EC concerning the performance of analytical methods and the interpretation of results.
LinkOut - more resources
Full Text Sources
Other Literature Sources
