Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites
- PMID: 26762460
- PMCID: PMC7614903
- DOI: 10.1038/nature16527
Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites
Abstract
Helminth parasitic infections are a major global health and social burden. The host defence against helminths such as Nippostrongylus brasiliensis is orchestrated by type 2 cell-mediated immunity. Induction of type 2 cytokines, including interleukins (IL) IL-4 and IL-13, induce goblet cell hyperplasia with mucus production, ultimately resulting in worm expulsion. However, the mechanisms underlying the initiation of type 2 responses remain incompletely understood. Here we show that tuft cells, a rare epithelial cell type in the steady-state intestinal epithelium, are responsible for initiating type 2 responses to parasites by a cytokine-mediated cellular relay. Tuft cells have a Th2-related gene expression signature and we demonstrate that they undergo a rapid and extensive IL-4Rα-dependent amplification following infection with helminth parasites, owing to direct differentiation of epithelial crypt progenitor cells. We find that the Pou2f3 gene is essential for tuft cell specification. Pou2f3(-/-) mice lack intestinal tuft cells and have defective mucosal type 2 responses to helminth infection; goblet cell hyperplasia is abrogated and worm expulsion is compromised. Notably, IL-4Rα signalling is sufficient to induce expansion of the tuft cell lineage, and ectopic stimulation of this signalling cascade obviates the need for tuft cells in the epithelial cell remodelling of the intestine. Moreover, tuft cells secrete IL-25, thereby regulating type 2 immune responses. Our data reveal a novel function of intestinal epithelial tuft cells and demonstrate a cellular relay required for initiating mucosal type 2 immunity to helminth infection.
Conflict of interest statement
Data deposition: not applicable
Reprints and permissions information is available at
The authors declare no competing financial interest.
Figures
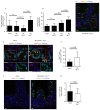

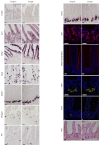



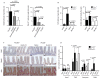


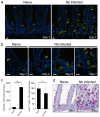
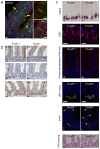
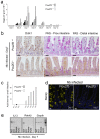
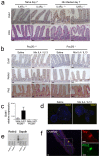
Comment in
-
Tuft Cells: A New Flavor in Innate Epithelial Immunity.Trends Parasitol. 2016 Aug;32(8):583-585. doi: 10.1016/j.pt.2016.04.016. Epub 2016 May 5. Trends Parasitol. 2016. PMID: 27161767
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

