Effect of Aging on Periodontal Inflammation, Microbial Colonization, and Disease Susceptibility
- PMID: 26762510
- PMCID: PMC4802783
- DOI: 10.1177/0022034515625962
Effect of Aging on Periodontal Inflammation, Microbial Colonization, and Disease Susceptibility
Abstract
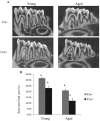
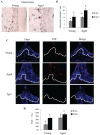
Periodontitis is a chronic inflammatory disease induced by a biofilm that forms on the tooth surface. Increased periodontal disease is associated with aging. We investigated the effect of aging on challenge by oral pathogens, examining the host response, colonization, and osteoclast numbers in aged versus young mice. We also compared the results with mice with lineage-specific deletion of the transcription factor FOXO1, which reduces dendritic cell (DC) function. Periodontitis was induced by oral inoculation of Porphyromonas gingivalis and Fusobacterium nucleatum in young (4 to 5 mo) and aged (14 to 15 mo) mice. Aged mice as well as mice with reduced DC function had decreased numbers of DCs in lymph nodes, indicative of a diminished host response. In vitro studies suggest that reduced DC numbers in lymph nodes of aged mice may involve the effect of advanced glycation end products on DC migration. Surprisingly, aged mice but not mice with genetically altered DC function had greater production of antibody to P. gingivalis, greater IL-12 expression, and more plasma cells in lymph nodes following oral inoculation as compared with young mice. The greater adaptive immune response in aged versus young mice was linked to enhanced levels of P. gingivalis and reduced bacterial diversity. Thus, reduced bacterial diversity in aged mice may contribute to increased P. gingivalis colonization following inoculation and increased periodontal disease susceptibility, reflected by higher TNF levels and osteoclast numbers in the periodontium of aged versus young mice.
Keywords: DNA-seq; bacteria; dendritic cell; lymphocyte; osteoclast; periodontitis.
© International & American Associations for Dental Research 2016.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures



Similar articles
-
FOXO1 deletion reduces dendritic cell function and enhances susceptibility to periodontitis.Am J Pathol. 2015 Apr;185(4):1085-93. doi: 10.1016/j.ajpath.2014.12.006. Am J Pathol. 2015. PMID: 25794707 Free PMC article.
-
Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: bone loss and host response.J Clin Periodontol. 2009 May;36(5):406-10. doi: 10.1111/j.1600-051X.2009.01393.x. J Clin Periodontol. 2009. PMID: 19419440
-
The function of dendritic cells in modulating the host response.Mol Oral Microbiol. 2018 Feb;33(1):13-21. doi: 10.1111/omi.12195. Epub 2017 Oct 9. Mol Oral Microbiol. 2018. PMID: 28845602 Free PMC article. Review.
-
Serum antibodies to Porphyromonas gingivalis chaperone HtpG predict health in periodontitis susceptible patients.PLoS One. 2008 Apr 23;3(4):e1984. doi: 10.1371/journal.pone.0001984. PLoS One. 2008. PMID: 18431474 Free PMC article.
-
Immunological Pathways Triggered by Porphyromonas gingivalis and Fusobacterium nucleatum: Therapeutic Possibilities?Mediators Inflamm. 2019 Jun 24;2019:7241312. doi: 10.1155/2019/7241312. eCollection 2019. Mediators Inflamm. 2019. PMID: 31341421 Free PMC article. Review.
Cited by
-
Pathogenic Molecular Mechanisms in Periodontitis and Peri-Implantitis: Role of Advanced Glycation End Products.Life (Basel). 2022 Jan 30;12(2):218. doi: 10.3390/life12020218. Life (Basel). 2022. PMID: 35207505 Free PMC article. Review.
-
Nisin, a Probiotic Bacteriocin, Modulates the Inflammatory and Microbiome Changes in Female Reproductive Organs Mediated by Polymicrobial Periodontal Infection.Microorganisms. 2024 Aug 12;12(8):1647. doi: 10.3390/microorganisms12081647. Microorganisms. 2024. PMID: 39203489 Free PMC article.
-
Aptamer Based SPREETA Sensor for the Detection of Porphyromonas gingivalis G-Protein.J Microbiol Biotechnol. 2024 Mar 28;34(2):289-295. doi: 10.4014/jmb.2310.10042. Epub 2023 Dec 18. J Microbiol Biotechnol. 2024. PMID: 38111313 Free PMC article.
-
Mucosal Immunity and the FOXO1 Transcription Factors.Front Immunol. 2019 Nov 29;10:2530. doi: 10.3389/fimmu.2019.02530. eCollection 2019. Front Immunol. 2019. PMID: 31849924 Free PMC article. Review.
-
Interconnections between Inflammageing and Immunosenescence during Ageing.Cells. 2022 Jan 21;11(3):359. doi: 10.3390/cells11030359. Cells. 2022. PMID: 35159168 Free PMC article. Review.
References
-
- Biddle A, Stewart L, Blanchard J, Leschine S. 2013. Untangling the genetic basis of fibrolytic specialization by lachnospiraceae and ruminococcaceae in diverse gut communities. Diversity. 5(3):627–640.
-
- Bodineau A, Folliguet M, Séguier S. 2009. Tissular senescence and modifications of oral ecosystem in the elderly: risk factors for mucosal pathologies. Curr Aging Sci. 2(2):109–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

