Loss of α-Tubulin Acetylation Is Associated with TGF-β-induced Epithelial-Mesenchymal Transition
- PMID: 26763233
- PMCID: PMC4777869
- DOI: 10.1074/jbc.M115.713123
Loss of α-Tubulin Acetylation Is Associated with TGF-β-induced Epithelial-Mesenchymal Transition
Abstract
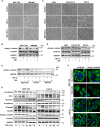
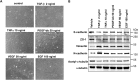
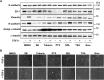
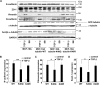
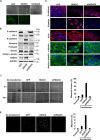
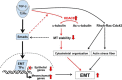
The epithelial-to-mesenchymal transition (EMT) is a process by which differentiated epithelial cells reprogram gene expression, lose their junctions and polarity, reorganize their cytoskeleton, increase cell motility and assume a mesenchymal morphology. Despite the critical functions of the microtubule (MT) in cytoskeletal organization, how it participates in EMT induction and maintenance remains poorly understood. Here we report that acetylated α-tubulin, which plays an important role in microtubule (MT) stabilization and cell morphology, can serve as a novel regulator and marker of EMT. A high level of acetylated α-tubulin was correlated with epithelial morphology and it profoundly decreased during TGF-β-induced EMT. We found that TGF-β increased the activity of HDAC6, a major deacetylase of α-tubulin, without affecting its expression levels. Treatment with HDAC6 inhibitor tubacin or TGF-β type I receptor inhibitor SB431542 restored the level of acetylated α-tubulin and consequently blocked EMT. Our results demonstrate that acetylated α-tubulin can serve as a marker of EMT and that HDAC6 represents an important regulator during EMT process.
Keywords: acetylation; epithelial-mesenchymal transition (EMT); histone deacetylase 6 (HDAC6); microtubule; transforming growth factor β (TGF-β).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

