Overlap of copper and iron uptake systems in mitochondria in Saccharomyces cerevisiae
- PMID: 26763345
- PMCID: PMC4736827
- DOI: 10.1098/rsob.150223
Overlap of copper and iron uptake systems in mitochondria in Saccharomyces cerevisiae
Abstract
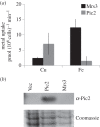
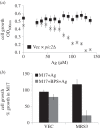
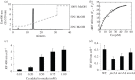
In Saccharomyces cerevisiae, the mitochondrial carrier family protein Pic2 imports copper into the matrix. Deletion of PIC2 causes defects in mitochondrial copper uptake and copper-dependent growth phenotypes owing to decreased cytochrome c oxidase activity. However, copper import is not completely eliminated in this mutant, so alternative transport systems must exist. Deletion of MRS3, a component of the iron import machinery, also causes a copper-dependent growth defect on non-fermentable carbon. Deletion of both PIC2 and MRS3 led to a more severe respiratory growth defect than either individual mutant. In addition, MRS3 expressed from a high copy number vector was able to suppress the oxygen consumption and copper uptake defects of a strain lacking PIC2. When expressed in Lactococcus lactis, Mrs3 mediated copper and iron import. Finally, a PIC2 and MRS3 double mutant prevented the copper-dependent activation of a heterologously expressed copper sensor in the mitochondrial intermembrane space. Taken together, these data support a role for the iron transporter Mrs3 in copper import into the mitochondrial matrix.
Keywords: copper; cytochrome c oxidase; iron; mitochondrial carrier family.
© 2016 The Authors.
Figures






References
-
- Hassett R, Dix DR, Eide DJ, Kosman DJ. 2000. The Fe(II) permease Fet4p functions as a low affinity copper transporter and supports normal copper trafficking in Saccharomyces cerevisiae. Biochem. J. 351, 477–484. (doi:10.1042/bj3510477) - DOI - PMC - PubMed
-
- Dancis A, Haile D, Yuan DS, Klausner RD. 1994. The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J. Biol. Chem. 269, 25 660–25 667. - PubMed
-
- Lin SJ, Pufahl RA, Dancis A, O'Halloran TV, Culotta VC. 1997. A role for the Saccharomyces cerevisiae ATX1 gene in copper trafficking and iron transport. J. Biol. Chem. 272, 9215–9220. (doi:10.1074/jbc.272.14.9215) - DOI - PubMed
-
- Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC. 2001. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem. 276, 38 084–38 089. (doi:10.1074/jbc.M105296200) - DOI - PubMed
-
- Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O'Halloran TV. 1999. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284, 805–808. (doi:10.1126/science.284.5415.805) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
