A Guide to Studying Human Hair Follicle Cycling In Vivo
- PMID: 26763421
- PMCID: PMC4785090
- DOI: 10.1038/JID.2015.354
A Guide to Studying Human Hair Follicle Cycling In Vivo
Erratum in
-
Erratum.J Invest Dermatol. 2016 Apr;136(4):883. doi: 10.1016/j.jid.2016.02.023. Epub 2016 Mar 21. J Invest Dermatol. 2016. PMID: 27140062 No abstract available.
Abstract
Hair follicles (HFs) undergo lifelong cyclical transformations, progressing through stages of rapid growth (anagen), regression (catagen), and relative "quiescence" (telogen). Given that HF cycling abnormalities underlie many human hair growth disorders, the accurate classification of individual cycle stages within skin biopsies is clinically important and essential for hair research. For preclinical human hair research purposes, human scalp skin can be xenografted onto immunocompromised mice to study human HF cycling and manipulate long-lasting anagen in vivo. Although available for mice, a comprehensive guide on how to recognize different human hair cycle stages in vivo is lacking. In this article, we present such a guide, which uses objective, well-defined, and reproducible criteria, and integrates simple morphological indicators with advanced, (immuno)-histochemical markers. This guide also characterizes human HF cycling in xenografts and highlights the utility of this model for in vivo hair research. Detailed schematic drawings and representative micrographs provide examples of how best to identify human HF stages, even in suboptimally sectioned tissue, and practical recommendations are given for designing human-on-mouse hair cycle experiments. Thus, this guide seeks to offer a benchmark for human hair cycle stage classification, for both hair research experts and newcomers to the field.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


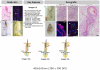

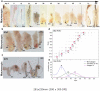
Comment in
-
Putting the Human Hair Follicle Cycle on the Map.J Invest Dermatol. 2016 Jan;136(1):4-6. doi: 10.1016/j.jid.2015.10.052. J Invest Dermatol. 2016. PMID: 26763414
References
-
- Al-Nuaimi Y, Hardman JA, Biro T, et al. A meeting of two chronobiological systems: circadian proteins Period1 and BMAL1 modulate the human hair cycle clock. The Journal of investigative dermatology. 2014;134:610–9. - PubMed
-
- Arck PC, Handjiski B, Kuhlmei A, et al. Mast cell deficient and neurokinin-1 receptor knockout mice are protected from stress-induced hair growth inhibition. J Mol Med (Berl) 2005;83:386–96. - PubMed
-
- Atanaskova Mesinkovska N, Bergfeld WF. Hair: what is new in diagnosis and management? Female pattern hair loss update: diagnosis and treatment. Dermatol Clin. 2013;31:119–27. - PubMed
-
- Bernard BA. The human hair follicle, a bistable organ? Experimental dermatology. 2012;21:401–3. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

