Mild systemic inflammation and moderate hypoxia transiently alter neuronal excitability in mouse somatosensory cortex
- PMID: 26763603
- PMCID: PMC4758894
- DOI: 10.1016/j.nbd.2015.12.019
Mild systemic inflammation and moderate hypoxia transiently alter neuronal excitability in mouse somatosensory cortex
Abstract
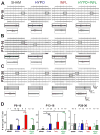
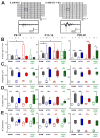
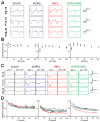
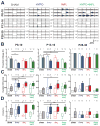
During the perinatal period, the brain is highly vulnerable to hypoxia and inflammation, which often cause white matter injury and long-term neuronal dysfunction such as motor and cognitive deficits or epileptic seizures. We studied the effects of moderate hypoxia (HYPO), mild systemic inflammation (INFL), or the combination of both (HYPO+INFL) in mouse somatosensory cortex induced during the first postnatal week on network activity and compared it to activity in SHAM control animals. By performing in vitro electrophysiological recordings with multi-electrode arrays from slices prepared directly after injury (P8-10), one week after injury (P13-16), or in young adults (P28-30), we investigated how the neocortical network developed following these insults. No significant difference was observed between the four groups in an extracellular solution close to physiological conditions. In extracellular 8mM potassium solution, slices from the HYPO, INFL, and HYPO+INFL group were more excitable than SHAM at P8-10 and P13-16. In these two age groups, the number and frequency of spontaneous epileptiform events were significantly increased compared to SHAM. The frequency of epileptiform events was significantly reduced by the NMDA antagonist D-APV in HYPO, INFL, and HYPO+INFL, but not in SHAM, indicating a contribution of NMDA receptors to this pathophysiological activity. In addition, the AMPA/kainate receptor antagonist CNQX suppressed the remaining epileptiform activity. Electrical stimulation evoked prominent epileptiform activity in slices from HYPO, INFL and HYPO+INFL animals. Stimulation threshold to elicit epileptiform events was lower in these groups than in SHAM. Evoked events spread over larger areas and lasted longer in treated animals than in SHAM. In addition, the evoked epileptiform activity was reduced in the older (P28-30) group indicating that cortical dysfunction induced by hypoxia and inflammation was transient and compensated during early development.
Keywords: Barrel cortex; Development; Electrophysiology; Epileptiform activity; Hypoxia; In vitro; Interleukin-1β; Mouse; Multi-electrode array; Systemic inflammation.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



Similar articles
-
Model of frequent, recurrent, and spontaneous seizures in the intact mouse hippocampus.Hippocampus. 2004;14(8):935-47. doi: 10.1002/hipo.20007. Hippocampus. 2004. PMID: 15390177
-
Comparison of spontaneous and evoked epileptiform activity in three in vitro epilepsy models.Brain Res. 2002 Aug 2;945(2):174-80. doi: 10.1016/s0006-8993(02)02751-8. Brain Res. 2002. PMID: 12126879
-
The afterhyperpolarizing potential following a train of action potentials is suppressed in an acute epilepsy model in the rat Cornu Ammonis 1 area.Neuroscience. 2012 Jan 10;201:288-96. doi: 10.1016/j.neuroscience.2011.11.008. Epub 2011 Nov 10. Neuroscience. 2012. PMID: 22100272
-
Involvement of NMDA and non-NMDA receptors in the neuronal responses of the primary motor cortex to input from the supplementary motor area and somatosensory cortex: studies of task-performing monkeys.Jpn J Physiol. 1998 Aug;48(4):275-90. doi: 10.2170/jjphysiol.48.275. Jpn J Physiol. 1998. PMID: 9757144
-
Osmolarity, ionic flux, and changes in brain excitability.Epilepsy Res. 1998 Sep;32(1-2):275-85. doi: 10.1016/s0920-1211(98)00058-8. Epilepsy Res. 1998. PMID: 9761327 Review.
Cited by
-
Cortical Gray Matter Injury in Encephalopathy of Prematurity: Link to Neurodevelopmental Disorders.Front Neurol. 2020 Jul 14;11:575. doi: 10.3389/fneur.2020.00575. eCollection 2020. Front Neurol. 2020. PMID: 32765390 Free PMC article. Review.
-
Can Neonatal Systemic Inflammation and Hypoxia Yield a Cerebral Palsy-Like Phenotype in Periadolescent Mice?Mol Neurobiol. 2019 Oct;56(10):6883-6900. doi: 10.1007/s12035-019-1548-8. Epub 2019 Apr 2. Mol Neurobiol. 2019. PMID: 30941732 Free PMC article.
-
Modulation of Neocortical Development by Early Neuronal Activity: Physiology and Pathophysiology.Front Cell Neurosci. 2017 Nov 29;11:379. doi: 10.3389/fncel.2017.00379. eCollection 2017. Front Cell Neurosci. 2017. PMID: 29238291 Free PMC article. Review.
-
Timed fetal inflammation and postnatal hypoxia cause cortical white matter injury, interneuron imbalances, and behavioral deficits in a double-hit rat model of encephalopathy of prematurity.Brain Behav Immun Health. 2024 Jul 5;40:100817. doi: 10.1016/j.bbih.2024.100817. eCollection 2024 Oct. Brain Behav Immun Health. 2024. PMID: 39188404 Free PMC article.
-
Early brain activity: Translations between bedside and laboratory.Prog Neurobiol. 2022 Jun;213:102268. doi: 10.1016/j.pneurobio.2022.102268. Epub 2022 Mar 29. Prog Neurobiol. 2022. PMID: 35364141 Free PMC article. Review.
References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (Barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Alefeld M, Sutor B, Luhmann HJ. Pattern and pharmacology of propagating epileptiform activity in mouse cerebral cortex. Experimental Neurology. 1998;153:113–122. - PubMed
-
- Battaglia G, Colciaghi F, Finardi A, Nobili P. Intrinsic epileptogenicity of dysplastic cortex: Converging data from experimental models and human patients. Epilepsia. 2013;54:33–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

