Association Patterns in Open Data to Explore Ciprofloxacin Adverse Events
- PMID: 26763627
- PMCID: PMC4704041
- DOI: 10.4338/ACI-2015-06-RA-0076
Association Patterns in Open Data to Explore Ciprofloxacin Adverse Events
Abstract
Background: Ciprofloxacin is one of the main drugs to treat bacterial infections. Bacterial infections can lead to high morbidity, mortality, and costs of treatment in the world. In this study, an analysis was conducted using the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (AERS) database on the adverse events of ciprofloxacin.
Objectives: The aim of this study was to explore unknown associations among the adverse events of ciprofloxacin, patient demographics and adverse event outcomes.
Methods: A search of FDA AERS reports was performed and some statistics was highlighted. The most frequent adverse events and event outcomes of ciprofloxacin were listed, age and gender specific distribution of adverse events are reported, then the apriori algorithm was applied to the dataset to obtain some association rules and objective measures were used to select interesting ones. Furthermore, the results were compared against classical data mining algorithms and discussed.
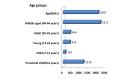
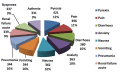
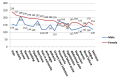
Results: The search resulted in 6 531 reports. The reports included within the dataset consist of 3 585 (55.8%) female and 2 884 (44.1%) male patients. The mean age of patients is 54.59 years. Preschool child, middle aged and aged groups have most adverse events reports in all groups. Pyrexia has the highest frequency with ciprofloxacin, followed by pain, diarrhoea, and anxiety in this order and the most frequent adverse event outcome is hospitalization. Age and gender based differences in the events in patients were found. In addition, some of the interesting associations obtained from the Apriori algorithm include not only psychiatric disorders but specifically their manifestation in specific gender groups.
Conclusions: The FDA AERS offers an important data resource to identify new or unknown adverse events of drugs in the biomedical domain. The results that were obtained in this study can provide valuable information for medical researchers and decision makers at the pharmaceutical research field.
Keywords: Data processing; adverse drug event; clinical care; clinical decision support; clinical informatics.
Conflict of interest statement
The author has no conflicts of interest to declare.
Figures
References
-
- Hrynaszkiewicz I. The need and drive for open data in biomedical publishing. Serials 2011; 24(1):31–37.
-
- Boulton G, Rawlins M, Vallance P, Walport M. Science as a public enterprise: the case for open data. Lancet 2011; 377: 1633–1635 - PubMed
-
- Poluzzi E, Raschi E, Piccinni C, De Ponti F. Analysis of the Publicly Accessible FDA Adverse Event Reporting System(AERS). Data Mining Applications in Engineering and Medicine 2012. http://www.intechopen.com/books/data-mining-applications-in-engineering-...
-
- Johnson KB, Lehmann CU, Council on Clinical Information Technology of the American Academy of Pediatrics. Electronic prescribing in pediatrics: toward safer and more effective medication management. Pediatrics 2013; 131(4):824–826. - PubMed
-
- Institute of Medicine Committeeon Quality in Healthcare in America. To Err is Human: building a Safer Health System. Washington DC: National Academies Press; 1999. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources