Folate-targeted pH-responsive calcium zoledronate nanoscale metal-organic frameworks: Turning a bone antiresorptive agent into an anticancer therapeutic
- PMID: 26763733
- PMCID: PMC4728024
- DOI: 10.1016/j.biomaterials.2015.12.018
Folate-targeted pH-responsive calcium zoledronate nanoscale metal-organic frameworks: Turning a bone antiresorptive agent into an anticancer therapeutic
Abstract
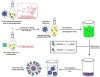
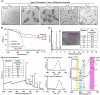
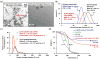
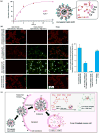
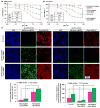
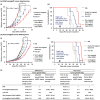
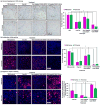
Zoledronate (Zol) is a third-generation bisphosphonate that is widely used as an anti-resorptive agent for the treatment of cancer bone metastasis. While there is preclinical data indicating that bisphosphonates such as Zol have direct cytotoxic effects on cancer cells, such effect has not been firmly established in the clinical setting. This is likely due to the rapid absorption of bisphosphonates by the skeleton after intravenous (i.v.) administration. Herein, we report the reformulation of Zol using nanotechnology and evaluation of this novel nanoscale metal-organic frameworks (nMOFs) formulation of Zol as an anticancer agent. The nMOF formulation is comprised of a calcium zoledronate (CaZol) core and a polyethylene glycol (PEG) surface. To preferentially deliver CaZol nMOFs to tumors as well as facilitate cellular uptake of Zol, we incorporated folate (Fol)-targeted ligands on the nMOFs. The folate receptor (FR) is known to be overexpressed in several tumor types, including head-and-neck, prostate, and non-small cell lung cancers. We demonstrated that these targeted CaZol nMOFs possess excellent chemical and colloidal stability in physiological conditions. The release of encapsulated Zol from the nMOFs occurs in the mid-endosomes during nMOF endocytosis. In vitro toxicity studies demonstrated that Fol-targeted CaZol nMOFs are more efficient than small molecule Zol in inhibiting cell proliferation and inducing apoptosis in FR-overexpressing H460 non-small cell lung and PC3 prostate cancer cells. Our findings were further validated in vivo using mouse xenograft models of H460 and PC3. We demonstrated that Fol-targeted CaZol nMOFs are effective anticancer agents and increase the direct antitumor activity of Zol by 80-85% in vivo through inhibition of tumor neovasculature, and inhibiting cell proliferation and inducing apoptosis.
Keywords: Cancer; Chemotherapy; Drug delivery; Folate-targeted nanoscale metal-organic frameworks; Zoledronate.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
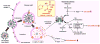
References
-
- Green JR. Bisphosphonates: preclinical review. Oncologist. 2004;9 Suppl 4:3–13. - PubMed
-
- Berenson JR, Rosen LS, Howell A, Porter L, Coleman RE, Morley W, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91:1191–200. - PubMed
-
- McKeage K, Plosker GL. Zoledronic acid: a pharmacoeconomic review of its use in the management of bone metastases. Pharmacoeconomics. 2008;26:251–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

