Sampling From the Proteome to the Human Leukocyte Antigen-DR (HLA-DR) Ligandome Proceeds Via High Specificity
- PMID: 26764012
- PMCID: PMC4824864
- DOI: 10.1074/mcp.M115.055780
Sampling From the Proteome to the Human Leukocyte Antigen-DR (HLA-DR) Ligandome Proceeds Via High Specificity
Abstract
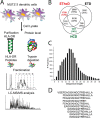
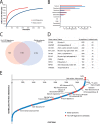
Comprehensive analysis of the complex nature of the Human Leukocyte Antigen (HLA) class II ligandome is of utmost importance to understand the basis for CD4(+)T cell mediated immunity and tolerance. Here, we implemented important improvements in the analysis of the repertoire of HLA-DR-presented peptides, using hybrid mass spectrometry-based peptide fragmentation techniques on a ligandome sample isolated from matured human monocyte-derived dendritic cells (DC). The reported data set constitutes nearly 14 thousand unique high-confident peptides,i.e.the largest single inventory of human DC derived HLA-DR ligands to date. From a technical viewpoint the most prominent finding is that no single peptide fragmentation technique could elucidate the majority of HLA-DR ligands, because of the wide range of physical chemical properties displayed by the HLA-DR ligandome. Our in-depth profiling allowed us to reveal a strikingly poor correlation between the source proteins identified in the HLA class II ligandome and the DC cellular proteome. Important selective sieving from the sampled proteome to the ligandome was evidenced by specificity in the sequences of the core regions both at their N- and C- termini, hence not only reflecting binding motifs but also dominant protease activity associated to the endolysosomal compartments. Moreover, we demonstrate that the HLA-DR ligandome reflects a surface representation of cell-compartments specific for biological events linked to the maturation of monocytes into antigen presenting cells. Our results present new perspectives into the complex nature of the HLA class II system and will aid future immunological studies in characterizing the full breadth of potential CD4(+)T cell epitopes relevant in health and disease.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Neefjes J., Jongsma M. L., Paul P., and Bakke O. (2011) Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 11, 823–836 - PubMed
-
- Adamopoulou E., Tenzer S., Hillen N., Klug P., Rota I. a, Tietz S., Gebhardt M., Stevanovic S., Schild H., Tolosa E., Melms A., and Stoeckle C. (2013) Exploring the MHC-peptide matrix of central tolerance in the human thymus. Nat. Commun. 4, 2039. - PubMed
-
- Suri A., Lovitch S. B., and Unanue E. R. (2006) The wide diversity and complexity of peptides bound to class II MHC molecules. Curr. Opin. Immunol. 18, 70–77 - PubMed
-
- Ovsyannikova I. G., Johnson K. L., Bergen H. R., and Poland G. A. (2007) Mass spectrometry and peptide-based vaccine development. Clin. Pharmacol. Ther. 82, 644–652 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

