Memory Stem T Cells in Autoimmune Disease: High Frequency of Circulating CD8+ Memory Stem Cells in Acquired Aplastic Anemia
- PMID: 26764034
- PMCID: PMC4744506
- DOI: 10.4049/jimmunol.1501739
Memory Stem T Cells in Autoimmune Disease: High Frequency of Circulating CD8+ Memory Stem Cells in Acquired Aplastic Anemia
Abstract
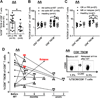
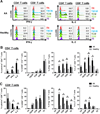
Memory stem T cells (TSCMs) constitute a long-lived, self-renewing lymphocyte population essential for the maintenance of functional immunity. Hallmarks of autoimmune disease pathogenesis are abnormal CD4(+) and CD8(+) T cell activation. We investigated the TSCM subset in 55, 34, 43, and 5 patients with acquired aplastic anemia (AA), autoimmune uveitis, systemic lupus erythematosus, and sickle cell disease, respectively, as well as in 41 age-matched healthy controls. CD8(+) TSCM frequency was significantly increased in AA compared with healthy controls. An increased CD8(+) TSCM frequency at diagnosis was associated with responsiveness to immunosuppressive therapy, and an elevated CD8(+) TSCM population after immunosuppressive therapy correlated with treatment failure or relapse in AA patients. IFN-γ and IL-2 production was significantly increased in various CD8(+) and CD4(+) T cell subsets in AA patients, including CD8(+) and CD4(+) TSCMs. CD8(+) TSCM frequency was also increased in patients with autoimmune uveitis or sickle cell disease. A positive correlation between CD4(+) and CD8(+) TSCM frequencies was found in AA, autoimmune uveitis, and systemic lupus erythematosus. Evaluation of PD-1, CD160, and CD244 expression revealed that TSCMs were less exhausted compared with other types of memory T cells. Our results suggest that the CD8(+) TSCM subset is a novel biomarker and a potential therapeutic target for AA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Risitano AM, Kook H, Zeng W, Chen G, Young NS, Maciejewski JP. Oligoclonal and polyclonal CD4 and CD8 lymphocytes in aplastic anemia and paroxysmal nocturnal hemoglobinuria measured by V beta CDR3 spectratyping and flow cytometry. Blood. 2002;100:178–183. - PubMed
-
- Nakao S, Takami A, Takamatsu H, Zeng W, Sugimori N, Yamazaki H, Miura Y, Ueda M, Shiobara S, Yoshioka T, Kaneshige T, Yasukawa M, Matsuda T. Isolation of a T-cell clone showing HLA-DRB1*0405-restricted cytotoxicity for hematopoietic cells in a patient with aplastic anemia. Blood. 1997;89:3691–3699. - PubMed
-
- Sloand E, Kim S, Maciejewski JP, Tisdale J, Follmann D, Young NS. Intracellular interferon-gamma in circulating and marrow T cells detected by flow cytometry and the response to immunosuppressive therapy in patients with aplastic anemia. Blood. 2002;100:1185–1191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

