Identification of DPY19L3 as the C-mannosyltransferase of R-spondin1 in human cells
- PMID: 26764097
- PMCID: PMC4803301
- DOI: 10.1091/mbc.E15-06-0373
Identification of DPY19L3 as the C-mannosyltransferase of R-spondin1 in human cells
Abstract
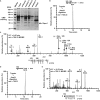
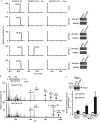
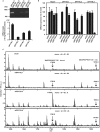
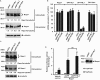
R-spondin1 (Rspo1) is a secreted protein that enhances Wnt signaling, which has crucial functions in embryonic development and several cancers. C-mannosylation is a rare type of glycosylation and might regulate secretion, protein-protein interactions, and enzymatic activity. Although human Rspo1 contains 2 predicted C-mannosylation sites, C-mannosylation of Rspo1 has not been reported, nor have its functional effects on this protein. In this study, we demonstrate by mass spectrometry that Rspo1 is C-mannosylated at W(153) and W(156). Using Lec15.2 cells, which lack dolichol-phosphate-mannose synthesis activity, and mutant Rspo1-expressing cells that replace W(153) and W(156) by alanine residues, we observed that C-mannosylation of Rspo1 is required for its secretion. Further, the enhancement of canonical Wnt signaling by Rspo1 is regulated by C-mannosylation. Recently DPY19 was reported to be a C-mannosyltransferase in Caenorhabditis elegans, but no C-mannosyltransferases have been identified in any other organism. In gain- and loss-of-function experiments, human DPY19L3 selectively modified Rspo1 at W(156) but not W(153) based on mass spectrometry. Moreover, knockdown of DPY19L3 inhibited the secretion of Rspo1. In conclusion, we identified DPY19L3 as the C-mannosyltransferase of Rspo1 at W(156) and found that DPY19L3-mediated C-mannosylation of Rspo1 at W(156) is required for its secretion.
© 2016 Niwa et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures


References
-
- Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13:11–26. - PubMed
-
- Buettner FF, Ashikov A, Tiemann B, Lehle L, Bakker H. C. elegans DPY-19 is a C-mannosyltransferase glycosylating thrombospondin repeats. Mol Cell. 2013;50:295–302. - PubMed
-
- Camp LA, Chauhan P, Farrar JD, Lehrman MA. Defective mannosylation of glycosylphosphatidylinositol in Lec35 Chinese hamster ovary cells. J Biol Chem. 1993;268:6721–6728. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

