Intracellular NAD+ levels are associated with LPS-induced TNF-α release in pro-inflammatory macrophages
- PMID: 26764408
- PMCID: PMC4770305
- DOI: 10.1042/BSR20150247
Intracellular NAD+ levels are associated with LPS-induced TNF-α release in pro-inflammatory macrophages
Abstract
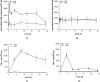
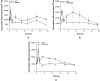
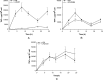
Metabolism and immune responses have been shown to be closely linked and as our understanding increases, so do the intricacies of the level of linkage. NAD(+) has previously been shown to regulate tumour necrosis factor-α (TNF-α) synthesis and TNF-α has been shown to regulate NAD(+) homoeostasis providing a link between a pro-inflammatory response and redox status. In the present study, we have used THP-1 differentiation into pro- (M1-like) and anti- (M2-like) inflammatory macrophage subset models to investigate this link further. Pro- and anti-inflammatory macrophages showed different resting NAD(+) levels and expression levels of NAD(+) homoeostasis enzymes. Challenge with bacterial lipopolysaccharide, a pro-inflammatory stimulus for macrophages, caused a large, biphasic and transient increase in NAD(+) levels in pro- but not anti-inflammatory macrophages that were correlated with TNF-α release and inhibition of certain NAD(+) synthesis pathways blocked TNF-α release. Lipopolysaccharide stimulation also caused changes in mRNA levels of some NAD(+) homoeostasis enzymes in M1-like cells. Surprisingly, despite M2-like cells not releasing TNF-α or changing NAD(+) levels in response to lipopolysaccharide, they showed similar mRNA changes compared with M1-like cells. These data further strengthen the link between pro-inflammatory responses in macrophages and NAD(+). The agonist-induced rise in NAD(+) shows striking parallels to well-known second messengers and raises the possibility that NAD(+) is acting in a similar manner in this model.
Keywords: TNF-α; immune responses; lipopolysaccharide (LPS); macrophages; pyridine nucleotides; second messenger.
© 2016 Authors.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

