Genetic and Metabolic Signals during Acute Enteric Bacterial Infection Alter the Microbiota and Drive Progression to Chronic Inflammatory Disease
- PMID: 26764594
- PMCID: PMC4839196
- DOI: 10.1016/j.chom.2015.12.006
Genetic and Metabolic Signals during Acute Enteric Bacterial Infection Alter the Microbiota and Drive Progression to Chronic Inflammatory Disease
Abstract
Chronic inflammatory disorders are thought to arise due to an interplay between predisposing host genetics and environmental factors. For example, the onset of inflammatory bowel disease is associated with enteric proteobacterial infection, yet the mechanistic basis for this association is unclear. We have shown previously that genetic defiency in TLR1 promotes acute enteric infection by the proteobacteria Yersinia enterocolitica. Examining that model further, we uncovered an altered cellular immune response that promotes the recruitment of neutrophils which in turn increases metabolism of the respiratory electron acceptor tetrathionate by Yersinia. These events drive permanent alterations in anti-commensal immunity, microbiota composition, and chronic inflammation, which persist long after Yersinia clearence. Deletion of the bacterial genes involved in tetrathionate respiration or treatment using targeted probiotics could prevent microbiota alterations and inflammation. Thus, acute infection can drive long term immune and microbiota alterations leading to chronic inflammatory disease in genetically predisposed individuals.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

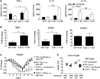



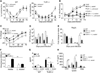
Comment in
-
Acute Gastroenteritis Leaves a Lasting Impression.Cell Host Microbe. 2016 Jan 13;19(1):3-5. doi: 10.1016/j.chom.2015.12.009. Cell Host Microbe. 2016. PMID: 26764589
References
-
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. - PubMed
-
- Autenrieth SE, Warnke P, Wabnitz GH, Lucero Estrada C, Pasquevich KA, Drechsler D, Gunter M, Hochweller K, Novakovic A, Beer-Hammer S, et al. Depletion of dendritic cells enhances innate anti-bacterial host defense through modulation of phagocyte homeostasis. PLoS Pathog. 2012;8:e1002552. - PMC - PubMed
-
- Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

