Inhibitory Effects of α-Lipoic Acid on Oxidative Stress-Induced Adipogenesis in Orbital Fibroblasts From Patients With Graves Ophthalmopathy
- PMID: 26765462
- PMCID: PMC4718288
- DOI: 10.1097/MD.0000000000002497
Inhibitory Effects of α-Lipoic Acid on Oxidative Stress-Induced Adipogenesis in Orbital Fibroblasts From Patients With Graves Ophthalmopathy
Abstract
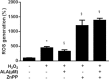
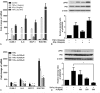
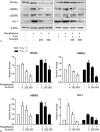
A choice of the optimal treatment for Graves ophthalmopathy (GO) is a challenge due to the complexity of the pathogenesis. Alpha-lipoic acid (ALA) is well known as a multifunctional antioxidant, helping to protect cells against oxidative stress and inflammatory damage.The aim of this study was to investigate the effects of ALA on intracellular production of reactive oxygen species (ROS), inflammation, and adipogenesis using primary cultured orbital fibroblasts from patients with GO.Intracellular ROS levels and mRNA expressions of proinflammatory cytokines and chemokines including intercellular adhesion molecule-1 (ICAM-1), interleukin (IL)-6, monocyte chemoattractant protein (MCP)-1, and regulated upon activation normal T cell expressed and presumably secreted (RANTES) were measured. After adipogenesis, the expressions of peroxisome proliferator-activated receptor (PPAR)γ, CCAAT-enhancer-binding proteins (C/EBP)α and β, and heme oxygenase-1 (HO-1) were investigated.H2O2 dose-dependently stimulated ROS production and HO-1 expression. Addition of ALA strongly attenuated ROS production and further increased HO-1 expression. However, by pretreatment of zinc protoporphyrin (ZnPP), HO-1 inhibitor, ALA inhibition of ROS generation by H2O2 was abolished. Tumor necrosis factor (TNF)α-induced mRNA expressions of ICAM-1, IL-6, MCP-1, and RANTES were inhibited by ALA treatment. In this context, TNFα-induced phosphorylation of P65 was also inhibited. In addition, ALA dose-dependently inhibited H2O2-induced intracellular accumulation of lipid droplets. The expression of adipogenic transcription factors, including PPARγ, C/EBPα, and β, was also inhibited.ALA is a potential therapeutic agent for GO because of the inhibitory effects on ROS production and gene expression of proinflammatory cytokines and chemokines, resulting in prevention of adipose-tissue expansion.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


References
-
- Korducki J, Loftus S, Bahn R. Stimulation of glycosaminoglycan production in cultured human retroocular fibroblasts. Invest Ophthalmol Vis Sci 1992; 33:2037–2042. - PubMed
-
- Bartalena L, Tanda ML, Piantanida E, et al. Oxidative stress and Graves’ ophthalmopathy: in vitro studies and therapeutic implications. Biofactors 2003; 19:155–163. - PubMed
-
- Burch HB, Lahiri S, Bahn RS, et al. Superoxide radical production stimulates retroocular fibroblast proliferation in Graves’ ophthalmopathy. Exp Eye Res 1997; 65:311–316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

