Alemtuzumab in T-cell large granular lymphocytic leukaemia: interim results from a single-arm, open-label, phase 2 study
- PMID: 26765645
- PMCID: PMC4721315
- DOI: 10.1016/S2352-3026(15)00227-6
Alemtuzumab in T-cell large granular lymphocytic leukaemia: interim results from a single-arm, open-label, phase 2 study
Abstract
Background: T-cell large granular lymphocytic leukaemia (T-LGL) is a lymphoproliferative disease that presents with immune-mediated cytopenias and is characterised by clonal expansion of cytotoxic CD3+ CD8+ lymphocytes. Use of methotrexate, ciclosporin, or cyclophosphamide as first therapy improves cytopenias in 50% of patients, but long-term use of these can lead to toxicity. We aimed to explore the activity and safety of alemtuzumab, an anti-CD52 monoclonal antibody, in patients with T-LGL.
Methods: We did this single-arm, phase 2 trial in consecutively enrolled adults with T-LGL referred to the National Institutes of Health in Bethesda, MD, USA. Alemtuzumab was given intravenously at 10 mg per day for 10 days. The primary endpoint was haematological response at 3 months after infusion. A complete response was defined as normalisation of all affected lineages, and a partial response was defined in neutropenic patients as 100% increase in the absolute neutrophil count to more than 5 × 10(8) cells per L, and in those with anaemia, as any increase in haemoglobin of 20 g/L or higher observed in at least two serial measurements 1 week apart and sustained for 1 month or longer without exogenous growth factors support or transfusions. Analysis was by intention to treat. We report results from the first stage of this Simon two-stage design trial; enrolment into the second stage is continuing. This study is registered with ClinicalTrials.gov, number NCT00345345.
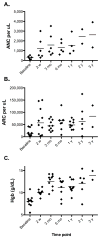
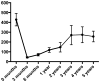
Findings: From Oct 1, 2006, to March 1, 2015, we enrolled 25 patients with T-LGL. 14 patients (56%; 95% CI 35-76) had a haematological response at 3 months. Four patients with associated myelodysplastic syndrome and two who had received haemopoietic stem cell transplantation had either no response or were not evaluable, meaning 14 (74% [49-91]) of the 19 patients with classic T-LGL responded. All patients had an infusion reaction (24 [96%] patients grade 1-2, one [4%] patient grade 3), which improved with symptomatic therapy. All patients developed lymphopenia, with 22 (88%) patients having grade 3 or 4 lymphopenia. The other most common grade 3 and 4 adverse events were leukopenia (eight [32%]) and neutropenic infections (five [20%]). Seven patients died; all were non-responders.
Interpretation: This is the largest and only prospective study of alemtuzumab in patients with T-LGL. The activity reported with a single course of a lymphocytotoxic drug in patients with mainly relapsed and refractory disease suggests that haematological response can be achieved without continued use of oral immunosuppression.
Funding: National Heart, Lung, and Blood Institute.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures





Comment in
-
Alemtuzumab in T-cell large granular lymphocyte leukaemia.Lancet Haematol. 2016 Jan;3(1):e4-5. doi: 10.1016/S2352-3026(15)00281-1. Epub 2015 Dec 17. Lancet Haematol. 2016. PMID: 26765647 No abstract available.
References
-
- McKenna RW, Parkin J, Kersey JH, Gajl-Peczalska KJ, Peterson L, Brunning RD. Chronic lymphoproliferative disorder with unusual clinical, morphologic, ultrastructural and membrane surface marker characteristics. The American journal of medicine. 1977;62(4):588–96. - PubMed
-
- Loughran TP, Jr, Kadin ME, Starkebaum G, et al. Leukemia of large granular lymphocytes: association with clonal chromosomal abnormalities and autoimmune neutropenia, thrombocytopenia, and hemolytic anaemia. Annals of internal medicine. 1985;102(2):169–75. - PubMed
-
- Pandolfi F, Loughran TP, Jr, Starkebaum G, et al. Clinical course and prognosis of the lymphoproliferative disease of granular lymphocytes. A multicenter study. Cancer. 1990;65(2):341–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

