Human Mesenchymal Stem Cells Genetically Engineered to Overexpress Brain-derived Neurotrophic Factor Improve Outcomes in Huntington's Disease Mouse Models
- PMID: 26765769
- PMCID: PMC4881765
- DOI: 10.1038/mt.2016.12
Human Mesenchymal Stem Cells Genetically Engineered to Overexpress Brain-derived Neurotrophic Factor Improve Outcomes in Huntington's Disease Mouse Models
Abstract
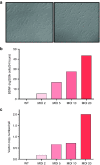
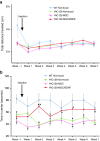
Huntington's disease (HD) is a fatal degenerative autosomal dominant neuropsychiatric disease that causes neuronal death and is characterized by progressive striatal and then widespread brain atrophy. Brain-derived neurotrophic factor (BDNF) is a lead candidate for the treatment of HD, as it has been shown to prevent cell death and to stimulate the growth and migration of new neurons in the brain in transgenic mouse models. BDNF levels are reduced in HD postmortem human brain. Previous studies have shown efficacy of mesenchymal stem/stromal cells (MSC)/BDNF using murine MSCs, and the present study used human MSCs to advance the therapeutic potential of the MSC/BDNF platform for clinical application. Double-blinded studies were performed to examine the effects of intrastriatally transplanted human MSC/BDNF on disease progression in two strains of immune-suppressed HD transgenic mice: YAC128 and R6/2. MSC/BDNF treatment decreased striatal atrophy in YAC128 mice. MSC/BDNF treatment also significantly reduced anxiety as measured in the open-field assay. Both MSC and MSC/BDNF treatments induced a significant increase in neurogenesis-like activity in R6/2 mice. MSC/BDNF treatment also increased the mean lifespan of the R6/2 mice. Our genetically modified MSC/BDNF cells set a precedent for stem cell-based neurotherapeutics and could potentially be modified for other neurodegenerative disorders such as amyotrophic lateral sclerosis, Alzheimer's disease, and some forms of Parkinson's disease. These cells provide a platform delivery system for future studies involving corrective gene-editing strategies.
Figures






References
-
- A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell 72: 971–983 (1993). - PubMed
-
- Huntington Study, G (2006). Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology 66: 366–372. - PubMed
-
- Killoran, A and Biglan, KM (2014). Current therapeutic options for Huntington's disease: good clinical practice versus evidence-based approaches? Mov Disord 29: 1404–1413. - PubMed
-
- Canals, JM, Pineda, JR, Torres-Peraza, JF, Bosch, M, Martín-Ibañez, R, Muñoz, MT et al. (2004). Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington's disease. J Neurosci 24: 7727–7739. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

