Class I and IIa HDACs Mediate HIF-1α Stability Through PHD2-Dependent Mechanism, While HDAC6, a Class IIb Member, Promotes HIF-1α Transcriptional Activity in Nucleus Pulposus Cells of the Intervertebral Disc
- PMID: 26765925
- PMCID: PMC4891304
- DOI: 10.1002/jbmr.2787
Class I and IIa HDACs Mediate HIF-1α Stability Through PHD2-Dependent Mechanism, While HDAC6, a Class IIb Member, Promotes HIF-1α Transcriptional Activity in Nucleus Pulposus Cells of the Intervertebral Disc
Abstract
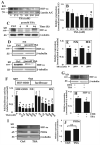
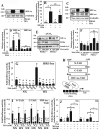
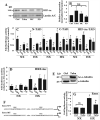
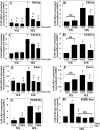
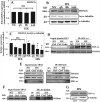
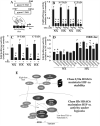
The objective of this study was to determine the role of histone deacetylases (HDACs) in regulating HIF-1α protein stability and activity in nucleus pulposus (NP) cells. Treatment of NP cells with pan-HDAC inhibitor TSA resulted in decreased HIF-1α levels under both normoxia and hypoxia in a dose-dependent fashion. TSA-mediated HIF-1α degradation was rescued by concomitant inhibition of not only the 26S proteasome but also PHD2 function. Moreover, TSA treatment of PHD2(-/-) cells had little effect on HIF-1α levels, supporting the notion that inhibition of PHD2 function by HDACs contributed to HIF-1α stabilization. Surprisingly, class-specific HDAC inhibitors did not affect HIF-1α protein stability, indicating that multiple HDACs controlled HIF-1α stability by regulating HIF-1α-PHD2 interaction in NP cells. Interestingly, lower-dose TSA that did not affect HIF-1α stability decreased its activity and target gene expression. Likewise, rescue of TSA-mediated HIF-1α protein degradation by blocking proteasomal or PHD activity did not restore HIF-1 activity, suggesting that HDACs independently regulate HIF-1α stability and activity. Noteworthy, selective inhibition of HDAC6 and not of class I and IIa HDACs decreased HIF-1-mediated transcription under hypoxia to a similar extent as lower-dose TSA, contrasting the reported role of HDAC6 as a transcriptional repressor in other cell types. Moreover, HDAC6 inhibition completely blocked TSA effects on HIF-1 activity. HDAC6 associated with and deacetylated HSP90, an important cofactor for HIF-1 function in NP cells, and HDAC6 inhibition decreased p300 transactivation in NP cells. Taken together, these results suggest that although multiple class I and class IIa HDACs control HIF-1 stability, HDAC6, a class IIb HDAC, is a novel mediator of HIF-1 activity in NP cells possibly through promoting action of critical HIF-1 cofactors. © 2016 American Society for Bone and Mineral Research.
Keywords: HDAC6; HIF-1α; HYPOXIA; INTERVERTEBRAL DISC; NUCLEUS PULPOSUS; TRANSCRIPTION FACTOR.
© 2016 American Society for Bone and Mineral Research.
Figures
References
-
- Gruber HE, Ashraf N, Kilburn J, et al. Vertebral endplate architecture and vascularization: application of micro-computerized tomography, a vascular tracer, and immunocytochemistry in analyses of disc degeneration in the aging sand rat. Spine (Phila Pa 1976) 2005;30:2593–600. - PubMed
-
- Rajpurohit R, Risbud MV, Ducheyne P, Vresilovic EJ, Shapiro IM. Phenotypic characteristics of the nucleus pulposus: Expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002;308:401–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

